Technology or Margins?
Where do you land?
Many articles I write are data driven. This one is more of a thought piece. That said, I think this pretty innocent question of “technology or margins?” translates quite well into a long-term important topic for our field to consider. Again, the goal for each piece here is maybe help even one or two adjust how they consider a common topic or theme within our specialty. And with that…
Originally drafted Jan 2024. Now published about 18 months later as it continues to resurface here and there and be a relevant issue.
A more recent trial made me head over to X and post this question:
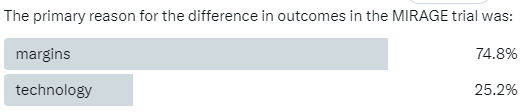
The primary reason for the difference in the outcomes in the MIRAGE Trial was?
MIRAGE Trial (context): This was a prostate cancer trial. The premise was to use an MRI Linac to treat prostate cancer with a slightly smaller margin. Using MRI, the ViewRay platform let you see the target live throughout the treatment delivery and automatically turned the beam on and off depending on the location of the target. Simply stated: the creators of the trial thought the visualization aided in margin reduction.
So they used tracking and MRI technology. But they also reduced the margins of the target in the “experimental arm”. And the trial did show that the patients in the MRI Linac arm had less toxicity.
The authors reported the trial primarily as a technology trial.
The technology was what safely allowed for the margin reduction in their opinion - read their words, listen to their podcasts. But on the contrary, more broadly, it was perceived to be a trial on margins - smaller margins result in less toxicity.
And today, a year later, I think the overwhelming majority of people still view this as a trial on margins. With, I think, the majority agreeing with the statement:
“I can get these same results with smaller margins on my own machine.” - My paraphrasing.
Today, we dive in and I’ll try to explain my perspective on why I think peoples views on this narrow trial speak so loudly to the future of our field; defining how we will make the push for “better”. In this particular trial, it is a tracking and MRI linac question, but I believe the larger fundamental issue for our field will be with regards to proton therapy and treatments that require high capital (MRI linac) or are very labor intensive (think adaptive approaches).
The argument for TECHNOLOGY:
A Simplistic Example: Shooting a target at a rifle range:
Scenario 1 is simply pulling the trigger every 5 seconds - yes - trying really hard and doing your absolute best, but end of the day, despite all your efforts to keep the thing “just right”, the gun goes off every 5 seconds.
Scenario 2 is just like above, except “AI” (because people love that term today), determines when you are on target and the computer fires the bullet determining a “correct” or “best” answer in the 5 second window.
Pretty easy to see that Scenario 2 is better. Not a perfect example, but a starting point.
Example 2: See what you treat
IF you could readily visualize and automatically beam on and beam off for any error level that you could choose - and it didn’t have a dollar cost part to the equation, which cases under beam for your clinic today - would you use it?
I believe it would be nearly every one, everyday, all the time. It might be 5mm in some cases and 2mm in others. Maybe even 1cm for a palliative case to help with “throughput”, but if it was free from a monetary standpoint and essentially automatic, it would be used routinely.
Look at IGRT implementation over the years: first it was used only on select cases, then a few times a week on tough cases. Now everywhere. We’d see the same thing.
And you can apply this exact scenario to adaptive treatments. If you remove logistics and time constraints - you almost certainly would adapt every time something “significant” changed. The main “variable” would be a customized tolerance based on site and risk moving to smaller and smaller tolerances with time.
The Opposing Argument for MARGINS:
It is quite dumb to think that medicine is as simple as my two examples above. Treating cancer is magnitudes more complex. And it lies within the most complex system ever known - the human body.
In the simplistic example above, you can precisely count and determine the exact number of hits and misses. 100% accuracy and 100% linkage between the shot result and target strike.
In our world of cancer treatment, it is FAR more complex. If we have a treatment, that for example would be 90% effective that trends towards 85% effective, we have to measure that 5%. But that 5% is buried within a literal sea of competing events - MIs, car wrecks, 2nd unrelated cancers, not to mention response rates, tumor heterogeneity, differences in hospital approaches etc. etc. And somewhere, there has to be some reasonable conversation on costs - we can’t afford to do everything for everyone - we need to focus on bigger picture items and do things that “truly move” the curve - we can’t constantly fiddle at the edges.
Even then, all of this assumes that there are not disadvantages to MRI such as a little less flexibility of MLC movement, and delivery time. After all, KISS “keep it simple stupid”. Fast good accurate treatment can mirror the outcomes of the “most advanced” approaches in real world applications - so long as the simple approach is done well. And realistically, at some point, even if theoretically “better”, complexity in and of itself adds risk. This isn’t theory; this is truth in any application.
Further in this particular example, there are, in fact, other tools (like the Sunnybrook rectal immobilization device - personally no thanks - haha) that can allow for this approach in a much more cost efficient manner. To this end, we have to optimize efficiency and costs on a more global perspective and balance that against small incremental possible improvements for one any individual. And therefore, the appropriate answer is margins.
***Please consider a like or a share or a comment. Content is free. Algorithms prefer paid, so this really does help the site reach people. Thanks in advance for your support!***
Background Context:
I have over-simplified each side purposefully but hopefully one can get the ideas of each from the two perspectives. And, to me, both have reasonable components and in places, both are quite valid. But I do ultimately land on technology.
Because, at the most basic level, everything we do requires technology. The essence of the benefit that I give to patients requires technology (no, not the EMR - haha, the radiation machine) and so, I guess, I’m biased. I have to have machines and software and CTs etc. etc. to impart basically all my benefit. Without the tech, I don’t offer much beyond counselling.
As I’ve long said:
Until I have x-ray vision and shoot radiation from my fingertips, I rely on technology.
Yet, the vast majority of physicians will land on margins:
But why? I believe it’s simply the easier to justify mental path. So we shift the goalposts slightly to “measurably different” from “which is probably the better approach”.
Consider this scenario: You work at a place that has everything - from protons to Cyberknife to MRI Linac to Truebeam - which would you pick for yourself in any case? That is quite a different standard from a demonstrable difference in a prospective trial of 150 patients.
One means you must refer more patients while the other creates a higher and higher bar for “others to prove” benefit.
This, of course, is my opinion. But when you dumbly sign up on Twitter back in the day with “protons” in your handle, you realize the danger in this space first hand. And for better or worse, my online experience has been ~99.9% consistent with the move to a proton center and watching / living through that transition from the view point of my current and prior practice. I’m convinced many people “choose” margin, because it is much easier to rationalize into their daily practice.
Margin means adjust. Technology means refer.
We all do more of the former.
Cyberknife: Another Current Example:
And even with my bias towards technology, I under sold it.
Prior to the release of PACE-B, with Cyberknife - I was wrongly convinced it wouldn’t matter.
(for anyone outside our field, Cyberknife “tracks” the target - staying on target throughout the delivery by moving the beam constantly to target).
I had succumb to the narrative. I was on record prior to the release of the PACE-B data that machine / technology would not matter. I had presented in great detail the history of our literature - where essentially all early intermediate risk SBRT prostate data was generated on Cyberknife machines, but I thought (especially with a high volume, large program enrolling on the linac side) that there would be no difference.
I thought that in prostate - where everyone does really well - that today, we would see no difference. That was my prediction. And even there, I wasn’t bullish enough on tracking and technology.
Because in PACE-B, Cyberknife vs. linac showed as a significant predictor benefit in reducing toxicity.
Large System Complexity: Perhaps a better argument against a referral.
I want to say that there are MANY things that we should consider alongside of technology - this is where, I believe many justifiably land when arguing against (or appearing to argue against) technology or not making referrals out of their hospital system.
How good are the physicians developing the plan? How good are the dosimetrists and physicists at optimizing the approach? How good are the surgeons and interventional radiologists you work with? How about the therapists and the safety protocols you have in place? What about systemic treatments and the oncology side of the equation where we are more integrated today than ever before? What about simple time delays in any referral?
And, it might be that singular question of how your program integrates with oncology is MORE valuable to patient outcomes than any stepwise improvements in technology your clinic might purchase on the radiation oncology side. I get that nuance.
This is at the heart of the historical argument for “complex care requires referral to some big center”. There likely is some validity to this argument for some disease sites - data seems to maybe support that. But I’ve also worked in a small community setting and understand both sides here. I could easily write an entire article on just this issue. But I do think this “system complexity” argument is one of the stronger retorts against referral for technology differences in some cases.
Ironically, much of the complexity related to a referral or more cooperative approach is purposefully created by growing administrative structures that seek to create institutional silos which are then rewarded when we choose “margins” and don’t refer.
Why Do I Keep Coming Back to this Question: margin vs. technology?
Because how you interpret the simple question of technology or margins generates two very distinct pathways on how you will respond in the future to data regarding proton therapy trials (or MRI Linac or Adaptive trials) that are nearing completion / publication.
Consider protons vs. photons: Take a child who needs craniospinal radiation for a childhood cancer - the opposite end of the spectrum from prostate cancer - purposefully.
The more you believe technology matters, the more one would refer that child to a proton center.
And between these two ends of the spectrum between prostate cancer and children requiring craniospinal, is a vast chasm of patients. And my guess is where you draw the line depends largely on a question very similar to how you view the MIRAGE trial.
Technology as the answer opposes the US Model
Variance in levels of technology actively conflicts with our administratively driven siloed institutional models. I believe that how we choose to move forward as a field regarding this issue is important to the structure and continued success of our field in producing value.
And this issue really hasn’t been pronounced in the US for decades. The goal for this site is write things that hold up 5 years later - that in hindsight remain relevant. We’ll see, but I believe technology differences with protons at the forefront are going to stir up the pot by continuing to show that with pencil beam outcomes are better in the highest 10% to maybe even 20% of cases and we have to figure out ways to use the resources more effectively.
Look at this pragmatic lung study out of the Netherlands and Italy. It’s a structure that I don’t believe is viable here in the US on a multi-institutional level. This lung study modeled predicted outcomes. It then picked the highest risk patients (ended up around 25% of the series) and sent them to be treated with protons. Not randomized, but prospectively collected consecutive cases with clear pre-specified stratification criteria.
I cover it in far greater depth in this prior article:
Spoiler: it worked. The higher risk cohort treated with protons had less toxicity - repeat - the ones predicted to do the worst, had less toxicity than the 75% treated with IMRT.
I actually think today, one can reasonably argue this type of structure makes more sense than true randomized studies within the proton world moving forward. We have a lot of trials in the works today that are true comparisons and we now have two randomized prospective trials (head and neck, and esophageal) (plus this consecutive series of lung patients) that have landed in favor of protons. (Obviously the older uniform scanning lung trial and prostate trial show no improvement - focus on more recent high risk patients treated with PBS - that is the current appropriate measuring stick).
In my view, the more we move towards this type of shared resource model today, the stronger our field will be in the future. Our field simply has too high of capital investment costs for everyone, everywhere to have all the tools. We need intelligent use trials and, more importantly, cooperative use institutional models.
Quick Aside:
Prostate Cancer vs. Esophageal Cancer
I was in a recent thread. And it was asked which cancer is most commonly treated with protons today in the US? Clearly a jab but I’m relatively thick skinned now 6 yrs in. The answer is, of course, prostate. Declining but still far too much of the proton market.
But the related, yet opposing, question could also apply.
How many esophageal cancers are treated within 10 miles of a proton center using IMRT since the publication of the Phase IIb data showing - nearly with 100% certainty (99.9%) - that protons have less toxicity?
Even if 1/2 of esophageal cancer cases (prior to ESOPEC/FLOT data) were referred to US proton centers, proton centers would be essentially full treating only kids and esophageal cancer. There would literally be no room for prostate cancer. (I estimate current national capacity is about 1.5%-2% of radiation cancer total capacity so filling that up takes very little marginal shifts in referral patterns).
So the answers to both questions is: too many.
Too many prostates with protons and too many esophageal cancers with photons. And therefore, I return to this question of margin or technology? And how do we maximize the resources we have for our patients? Because, while the MIRAGE trial was an MRI linac question, the basic question also applies to adaptive planning. It is a live imaging tracking or automatic gating question. And yes, it likely is a proton question. Long gone is the decade or two where basically everyone had access to a “TrueBeam” and all had the same tools.
An Attempt at a Summary:
Obviously, you’re on a proton site (see url / site title) - but I really do try to be balanced and data driven. Clearly I have biases. If you read much at all here, you realize that I favor technology as a critical component of our field. Ultimately I still think protons will show benefits in high risk cases - I’ve said some 10% or so will clearly and demonstrably benefit - I’ve remained steady in that belief now for about 8 years.
But consider this. In the last few years alone I believe we had numerous examples of technology clearly making a difference in outcomes:
PACE-B: Cyberknife arm had lower toxicity.
MIRAGE: MRI Linac arm had lower toxicity.
Lung Hematologic Study: Protons showed less toxicity.
We live today in a world where many seem to have decided that today’s equipment from a technology standpoint is “good enough” - my words. Looking out just a few years, I’m still strongly opposed to this view. To the contrary, my argument is much more that nothing we do today is good enough in our management of future cancer patients.
Is there a hazard rate argument to be made? At some point is good enough, good enough? Maybe… Kind of…
I understand that - as machines improve, you get to a point where there is potentially less and less value to each extra dollar spent. I get that. But the point on the curve where that happens will also move. If you back up and compare your current technology to Cobalt machines, all would agree where in a much better spot today. But it took us decades of work to move from Cobalt to linacs to IMRT to SBRT. Decades.
And when you view the change on this level - over decades - the incremental creep of technology becomes undeniable in its effect.
One change here or one change there might not seems to add up to much, but over time - combined, they are the foundation of what we are able to achieve today.
It takes time. Costs and complexity of the design had to improve and come down. The integration of stronger computers and better algorithms had to be implemented but there is no going back. In the future, I have zero doubt, we’ll track the tumors live for nearly everything and in a lot of cases, we’ll use the best beam paired with rapidly adapted and re-optimized plans. This is simply where we are headed.
I guess I could be wrong. Every future “breakthrough” could be similar to the ESOPEC esophageal trial where more chemotherapy (or via immunotherapies) move the bar soooo far that radiation is then excluded from future treatment pathways. Ironically, I think the more we don’t push on technology as a path forward for our field, this likelihood increases - a self-fulfilling prophesy of sorts. Looking back over my career, I’m certain that we would have far less use-cases today if we remained in a 3D SSD treatment world.
And almost reflexively therefore I land on technology.
In 10 years:
In 10 years, we’ll visually track nearly all tumors during the entirety of the treatment cycle. We’ll give higher and higher doses to more conformal targets - smaller and smaller “PTVs” and more precise “GTV” and “CTV” definitions. We’ll do those higher dose treatments in fewer and fewer fractions. AI will help largely diminish differences in experience and planning optimization. AI will be re-contouring and adapting the highest risk OARs and highest risk cases much more routinely. I’m hopeful AI will assist with our modeling to select which patients benefit the most by examining machine properties - photons, protons, and maybe even carbon - to get patients to the best spot for their treatment.
“With quantum computers in the cloud using AI to select which patients benefit the most” - sounds like a movie script - way far off, but likely far sooner than most consider today. And with these shifts, outcomes will improve. At least these are my hopes.
I remain incredibly optimistic. Even if we struggle to accurately parse referrals among institutions to the technologies and institutions where they benefit the most, we’ll learn faster and push towards better outcomes more efficiently through the impact of fancier targeting and more precise delivery of dose to the area we want it to go.
But I do think leadership, especially within regional areas, need to look beyond their walls and figure out effective business partnership arrangements that allow an efficient flow of patients to the best technology match for different disease sites. These types of business arrangements take time - often years. And, unfortunately, I believe the data and reality of this shift are on a similar, if not shorter, timeframe. The clock is ticking - quite loudly from my vantage point.
This isn’t about an institution or modality “winning”, this is about pushing aggressively for the best outcomes for the patient you are seeing today and tomorrow. I believe in the next decade, for better or worse for our field, technology will play a larger role in this calculation and we must prepare for this reality today. We talk a lot about “individualized care” in oncology. For the field of radiation oncology, I do think that carries a slightly different perspective - matching expensive relatively “scarce” technology to the disease. And that is one I believe our field (at least in the US) is struggling to appropriately get ahead of. From my viewpoint, we must begin to better address how we best utilize and share resources for our patients, and work to break down the institutional walls in the US that create massive barriers to a more cooperative care approach. I believe that is an important aspect of our path to better and that is why I circled back to this today some 18 months post the initial draft.
Something to consider. Thoughts of one.
As always - keep pushing for better and thanks for reading along.





Great commentary, Mark. As always, insightful and thoroughly thought out.
There is debate as to what drives human progress. Some say inventions (technology), others say "paradigm shifts" - e.g. the heliocentric Copernican model of the solar system.
I think it's both, but that technology comes first. Without telescopes and precise measuring instruments in the hands of scientists, the old astronomical models (akin to sloppy wide margins) could not be rejected.
One of the ongoing criticisms of proton therapy regards its proliferation despite the lack of randomized trial data. What this ignores though, is that a good, large clinical trial requires a multi-site infrastructure to design and conduct it, and even then it takes over a decade for enough patients to accrue and be followed for 5-10 years. Insurance denials, referral/ reimbursement patterns and lack of geographic access remain barriers to accrual for common indications, at least in the US.
Once we have good technology (IMPT), widely distributed enough for large scale research to occur, then enough data can accumulate for paradigm shifts to follow (or not).
Keep up the good work!