Prostate Cancer: An SBRT Journey: Part 2: Intermediate Risk Outcome Data
Based on my own experiences, we'll look closer at SBRT intermediate risk outcome data
In Part 1, I described my personal experiences with SBRT. As we discussed, I worried a bit about PSA kinetics post-treatment and thought I saw a bump in toxicity. I thought that clinically, both of these were different than my expectations. But I’m an engineer and data driven, so I started my own database that now has around 250 patients. It still is early and has rather limited follow-up, but it does appear to demonstrate PSA kinetics being significantly different - even when using SBRT doses on the higher side recommended (38 or 40 Gy - not 36.25).
As I said, I don’t have the answers - I’m looking for them. Step one for me was to look critically at the data and see just how out of line what I see is or is not. So today, we’re going to look across pretty big data sets and evaluate what I see in our data for intermediate risk disease, hopefully covering most of our prospective datasets. I agree with the tone and presentation in some publications and disagree with the conclusions in others. As always, you decide where you fall.
I do not care one bit if SBRT is the answer. I simply want the best answer for the most men in the most generalized settings and I want there to be good conversation happening between physicians and patients regarding expectations for various approaches and risk / benefits of different approaches.
Reminder: This is intermediate risk disease discussion. Low risk data shows excellent results - today, we just don’t treat that much low risk disease.
The SBRT 6000 Patient Meta-Analysis
I’ll start with where I happened to start, the 6000 patient Meta-Analysis. Easy to find, highly referenced. Google led me here.
Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies (ref 1) was published back in April of 2019.
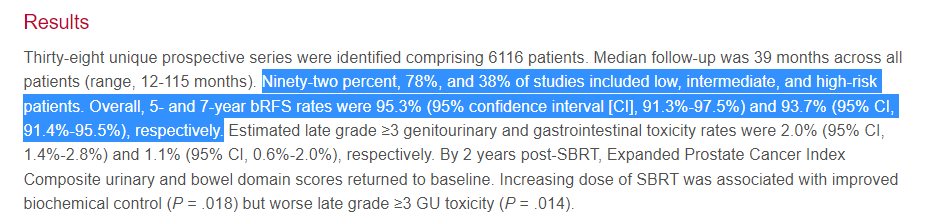
This is a large meta-analysis that looked at basically all prospective data and concluded SBRT results in a 95.3% bRFS at 5 yrs and argues that SBRT as of 2019 represents a standard of care option. Here are the results - straight from the abstract - please read / re-read it in entirety for context:
Moving on to table 1, you see the results presented here - 95.3% bRFS.
Honestly, this paper doesn’t sit well with me. The “headlines”, to me, present global results of 95% across a broad cohort of prostate cancer patients. Per abstract, 78% and 38% of studies include intermediate and high risk patients and in the next sentence, claims 95% control - at least that is what I see being advertised.
It’s consistent with what I “thought” I knew, but not consistent with what I was seeing in my clinic. In contrast to the headlines, it doesn’t take long to compare Table 1 to the abstract and see significant difference: bRFS is 2000+ rather than 6000 patients with about 79 TOTAL high risk patients. Well, if the abstract appears that different from Table 1, then what did the individual trials show.
I’ll just summarize by stating I disagree that the data in Table 1 supports outcomes of bRFS at 95% for disease that is greater risk than low risk prostate cancer.
An early draft of this Substack article goes trial by trial in pretty deep detail through the entire outcome aspect of the meta-analysis to illustrate my issues with the conclusions and how poorly supported (my assessment) the abstract is with regard to the statements it makes regarding outcomes. In the end, I’ve decided to focus elsewhere and move faster through my review.
Where is the review helpful? A starting list of some really good trials:
Specifically: Katz (ref 2), Meier (ref 3), Fuller (ref 4), and the Widmark(ref 5) data.
The first 3 are Cyberknife trials and in total contain over 430 intermediate risk patients. Meier is by far the strongest with intermediate risk at ~95% bRFS - it is single best trial of the 10 consistent with the abstract stating that SBRT achieves 95% bRFS in non-low risk men in my assessment. Katz is right at 90% for intermediate risk disease and Fuller on a longer follow-up (ref 6) achieves 92% for favorable and 83% for unfavorable intermediate risk (in the Meta-analysis, the follow-up was shorter and presented at 92% for IR disease as a broad category). These three trials arguably show IR somewhere in a 90%-95% range - but likely closer to 90% - you decide.
Widmark data: HYPO-RT-PC trial is the only randomized prospective dataset. It is larger than any other dataset, higher risk but still almost all IR (89%) and it achieves 83.7% control (which is less than 95%).
The remaining IR disease patients from the table within Linac programs come from three trials: Mantz (ref 7) allowed some 3+4 disease with <25% biopsies involved (so 33 men with very FIR disease) treating to 40 / 5 - results not broken down by risk group but realistically no one failed. Results reported for the trial at 99% bRFS. Arguably this would be the second trial that has some support of 95% or greater bRFS for generalized intermediate risk disease - you can decide how much weighting it deserves. Alayed (ref 8) is 12 men with IR disease - we will instead review a bigger series from this institution in just a moment. Boike (ref 9) is a dose escalation trial to 45/47.5/50 - doses that caused toxicity and aren’t recommended today.
As for high risk, we have two trials plus a SINGLE additional patient. Widmark is globally at 83.7% including mainly IR so one would expect HR should be below that number. And a second study from Lee (ref 10) that reports bRFS of 89.7% but if you read the paper, the patients actually had a disease free survival of 71% due to clinical progression and metastatic cases. Leaving one patient in the Alayed trial not reviewed.
Like I said, I don’t believe the data supports the abstract on treatment expectations beyond low risk disease. If I missed something, please comment.
With that, I was frustrated.
But perhaps there was stronger data with more realistic conclusions. Medical literature is simply huge today and tracking down lots of different sources is difficult. When you’ve been out in private practice and not writing for years, it is difficult to gain context for deep details if it hasn’t been your focus for years. Surely this can’t be right?…
And it isn’t. There are other data points to consider beyond the scope of the meta-analysis so we dig deeper.
It is 2142 men from 10 single institution trials from just earlier in 2019 - published 2 months prior to the above analysis. Median follow-up was 6.9 years. Per abstract, FIR is bDFS is 91.4% 7yr and 85.1% for UIR. Failure rates were Phoenix definition. All prospective patients.
Overall, it is high quality data and the abstract represents results for IR disease in the upper 80s (just below 90%). I can’t reconcile some small items like: 12.4% of men with UIR in the abstract compared to 9.9% in table one, but again - strong data - Google should have led me here.
To me, this is the gold standard dataset / paper arguing in favor of SBRT.
Conclusions and Relevance: (from the Consortium Study)
In this study, stereotactic body radiotherapy for low-risk and intermediate-risk disease was associated with low rates of severe toxic events and high rates of biochemical control. These data suggest that stereotactic body radiotherapy is an appropriate definitive treatment modality for low-risk and intermediate-risk prostate cancer.
Great study - amazing reference dataset. It supports an bRFS is just below 90% for intermediate risk disease with rational and appropriate conclusions. Very low nadirs - excellent kinetics. Consistent with ~90% in my view - NOTE: Not 95%, but 88% to 93% - likely dependent on dose.
(Most of this same data is then supported further in the more recent Ma study, Refining the definition of biochemical failure in the era of stereotactic body radiation therapy for prostate cancer: The Phoenix definition and beyond. (ref 12) < this paper is a great source of PSA kinetics for SBRT - a reference paper to me - largely the same patient population.)
A note on Cyberknife: In the Consortium data, there are 2142 patients of whom 35% are intermediate risk (so 750 men). In that intermediate risk subset, a grand total of 12 patients are treated on a Linac out of Sunnybrook.
And if you want to look up above to the meta-analysis, beyond Widmark, there are around 100 total men treated with linac based treatment. Most of those 100 men (about 60) were treated on a trial that caused significant toxicity and doses now aren’t recommended. On the Widmark trial, for essentially IR risk disease, disease free survival was 83.7% which appears significantly lower than most prospective data.
Did you realize that?
But there is additional linac prospective outcome data - not a ton mind you, but it largely lies beyond these two large sources. Sunnybrook has been a leader for decades in SBRT Linac based treatment. They have published a number of prospective SBRT trials using linacs. Generally the studies are smaller prospective trials advancing their internal process. In both the meta analysis and the Consortium data, this program is represented by the “Alayed” authorship or for low risk earlier series, “Loblaw”. (so it IS represented, just rather small representation due to their trial structure).
They have published a great history of their program which I recommend that you can find here (ref 13). Of their studies, the one I see as most pertinent to IR disease is the PATRIOT Trial. (I believe it to be the largest collection of IR disease by far that I found from their institution).
PATRIOT Trial (ref 14): This is a later larger and stronger intermediate risk dataset out of Sunnybrook. It came out after both of the above papers but instead of just 30 patients with 12 being IR or HR, this has ~152 IR patients, so we’ll cover it.
(They do have other trials that push the total number of IR patients above 200 within prospective trials < that is my best estimate - but they lay in small cohorts across numerous studies. But yes, they do have other cohorts of IR disease in prospective published series).
For the PATRIOT Trial, mean follow-up was 62 mos - a fid based program with linac treating to a bit higher dose of 40 / 5. It is better known as the every other day vs. once per week SBRT trial.
It reports low nadirs with bRFS presented at ~95%. It showed that cure rate didn’t change but spreading out the course of treatment helped lessen toxicity. Per Dr. Loblaw, they current recommend QOD as routine. There were a few more failures in the once per week arm but the p value was not significant between every other day and once a week - apparently there is an update of this trial coming later this year.
So that is pretty easy to remember: The single largest trial for linacs in intermediate risk disease in our literature is the one where they compared every other day treatment to once a week treatment. It’s out of Sunnybrook (not Sunnyvale, CA where the Cyberknife is made). They found no difference in cure rates between the two approaches. The once a week approach resulted in better bowel and bladder quality of life scores, but then ultimately no one uses once a week treatment and they reverted back to every other day in their own program. Got it. (note: once PACE B is reported, this piece of ironic trivia will be history - use it now or never).
Look to future: PACE B and NRG005
In the end, we have to reconcile the prospective randomized data of 83.7% results with the meta-analysis abstract / PATRIOT Trial results of 95% or even >99% out of Mantz and the beaches of Fort Meyers. One of these will be more correct for intermediate risk, two will be less so. In my opinion, we should / will likely see a dose dependent response from 36 - 40 in 5 fractions for intermediate risk cohorts. Based on EQD2 and the Widmark data, I don’t think 3625 is sufficient to reach 90% control for intermediate risk disease.
Take just the HYPO-RT-PC and PATRIOT trials. Yes they are different cohorts, but as you see below, they are not THAT different. HYPO-RT-PC is a bit higher risk, but not near to the extent of difference in outcomes - at least by my eye - you judge - remember 83.7% for HYPO-RT-PC vs. 95% for PATRIOT.
What are our options as the primary driver of this difference?
The best argument seems to be a dose dependent difference in cure rate between the two ends of our own NCCN guidelines - that 3625 / 5 gets us hopefully into the low to mid 80s and 4000 /5 gets us above 90%. If this is the main factor, then I’d point to NCCN Guidelines and ask why recommend such a large dose gradient? If we see this in our data, perhaps the check box next to intermediate and high risk should be reconsidered for marginal doses. As I have argued repeatedly, if dose matters, it should be reflected in the guidelines.
I think a close second is that a single high volume institution understands the nuance of treatment and these results are consistent with what Sunnybrook achieves with their linac program with decades of experience. To me, the data suggests more programs seem able to reproduce high quality outcomes easier in Cyberknife settings. This would then argue that when you roll out SBRT to multiple institutions, there is still quite a learning curve. Like the first option, this carries broader ramifications to our treatment standards and expectations.
And finally my guess is patient mix. I think this is actually a distant third as the most likely difference between these two published series. They are different and maybe somehow those apparently pretty subtle differences account for the majority of difference we see, but I don’t think so.
Ultimately, We Need Data:
So trials are needed. And fortunately two are on the way.
PACE-B (ref 15): It compares 3625 / 5 fractions to 78 / 39 fractions. The 78 / 39 fraction approach can be altered to 62 / 20 fraction approach (note the dose bump from 60/20). So two standard arm doses vs. SBRT. It is a non-inferiority design. The anticipated DFS for a favorable subset of IR is 85%. Depending on final enrollment, the trial will accept approximately a 6% difference in control rates - so the pre-trial goal is superior to 79% as a valid confirmation of the approach. This trial is closed to accrual and is LR(7%) / IR (93%) (non-4+3) disease. Note: it has good blend of non-Cyberknife and Cyberknife patients - not randomized, but it should add to the data supporting little difference in current times between these two machine approaches.
NRG005 (ref 16): The trial seeks to demonstrate that 3625 is “superior” to 70 / 28 fractions. There are two arms to the primary objective GU and GI toxicity and then DFS. It is interesting as it is a dose AND margin trial 8mm / 5mm for standard fractionation (larger than I have used in 15 yrs) vs. 5mm / 3mm for SBRT which is much more in line with most practices today (my assessment). I can’t find as good of data on the power / statistical calculations for this trial but it is also closed to accrual.
My Summary:
Intermediate data and expectations are mixed.
Most on Twitter believe PACE will beat pre-trial anticipated outcomes used in the power calculations by more than 7%. I tend to go much closer to the trial as designed - say 87.5% for 3625 in more favorable intermediate risk disease. If you were to move towards more unfavorable disease, I believe we will need 40/5 type dose / approaches to attain near 90% disease free survival. If stay with more moderate dose like 3625/5 in more unfavorable risk disease, that number will fall well below 85%. I think those statements are more representative of data up to today - we’ll see.
One final word on “Cyberknife”:
When I posted that word on Twitter, it appeared to cause a reaction similar to the word “proton” online. I didn’t expect that. Do I think that today there is a large difference between a high volume Cyberknife SBRT program and a high volume Linac program? No I do not. Decades of experience matter.
I’m very pleased that PACE-B will largely answer this question even if not via a planned endpoint. It won’t be perfect but in a randomized controlled trial, it will be very good data - and honestly if Sunnybrook contributes meaningful numbers to the trial on the linac side, this will nearly guarantee equivalence - they simply are a top tier program with decades of experience.
But do I think that we should assume there is no benefit to tracking of the disease during delivery and consistency of programs due to a machine technology - no. Furthering my argument, linacs just lost in a trial comparing toxicity to MRI Linacs (ref 17).
I understand people like to then quote the MIRAGE trial as: (my paraphrasing)
the difference in outcomes are due to different planning margins rather than machine / technology / investment.
But the principle investigator believes it was precisely machine / technology / investment that allowed him to decrease those margins - ie knowing where the tumor is and tracking it helped safely shrink margins.
Oh, and even in PACE B, this data was presented in 2021 by Alison Tree at ESTRO 2021 showing less GI/GU toxicity with Cyberknife.
That’s why I think it is important to mention, even if the era when it was really helping us move forward has passed. It is part of our history and active tracking of the target is going to help on some level (I think nearly negligible in prostate). As I’ve said, the opportunity for Cyberknife to prove benefit over linac was at least a decade ago. Linacs have improved. But at the same time, I don’t think it is unreasonable for a patient who lives in the middle of the country to seek out a Cyberknife machine over a low volume linac SBRT machine program. Maybe PACE will change that assessment, maybe not. We’ll follow the data.
And with that, I believe we have covered data supporting SBRT use in intermediate risk disease. Maybe now you have a different context to view the literature - different things to look for in the upcoming trials and maybe a different view of our history. If so, subscribe or share the blog. I appreciate the support.
In the next article, we’ll look at a the data a bit closer. How we tend to move goalposts and I’ll explain why it makes perfect sense that the 5 yr Fuller bDFS data declined nearly a full 5% with longer follow-up. We’ll look at how we calculate comparative doses and what we count as failures. If we just painted with a broad brush, next we’ll dive into the weeds.
REFERENCES:
Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies
https://www.redjournal.org/article/S0360-3016(19)30612-1/fulltext#back-bib61Stereotactic body radiotherapy as treatment for organ confined low- and intermediate-risk prostate carcinoma, a 7-year study - Katz
DOI: 10.3389/fonc.2014.00240Multicenter Trial of Stereotactic Body Radiation Therapy for Low- and Intermediate-Risk Prostate Cancer: Survival and Toxicity Endpoints - Meier - Cyberknife data
https://pubmed.ncbi.nlm.nih.gov/30191864/Virtual HDR CyberKnife SBRT for Localized Prostatic Carcinoma: 5-Year Disease-Free Survival and Toxicity Observations - Fuller
DOI: 10.3389/fonc.2014.00321Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial - Widmark
DOI: 10.1016/S0140-6736(19)31131-6High Dose “HDR-Like” Prostate SBRT: PSA 10-Year Results From a Mature, Multi-Institutional Clinical Trial
https://www.frontiersin.org/articles/10.3389/fonc.2022.935310/fullA Phase II Trial of Stereotactic Ablative Body Radiotherapy for Low-Risk Prostate Cancer Using a Non-Robotic Linear Accelerator and Real-Time Target Tracking: Report of Toxicity, Quality of Life, and Disease Control Outcomes with 5-Year Minimum Follow-Up - Mantz
DOI: 10.3389/fonc.2014.00279Dose escalation for prostate stereotactic ablative radiotherapy (SABR): Late outcomes from two prospective clinical trials - Alayed
DOI:https://doi.org/10.1016/j.radonc.2018.03.005Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer - Boike
https://pubmed.ncbi.nlm.nih.gov/21464418/Stereotactic body radiation therapy for prostate cancer patients with old age or medical comorbidity: a 5-year follow-up of an investigational study - Lee SW
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4603131/Long-term Outcomes of Stereotactic Body Radiotherapy for Low-Risk and Intermediate-Risk Prostate Cancer
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2723641Refining the definition of biochemical failure in the era of stereotactic body radiation therapy for prostate cancer: The Phoenix definition and beyond
https://pubmed.ncbi.nlm.nih.gov/34774650/Evolution of hypofractionated accelerated radiotherapy for prostate cancer - the Sunnybrook experience. Loblaw
https://cyberleninka.org/article/n/1159482/viewerAccelerating prostate stereotactic ablative body radiotherapy: Efficacy and toxicity of a randomized phase II study of 11 versus 29 days overall treatment time (PATRIOT)
https://pubmed.ncbi.nlm.nih.gov/32416376/PACE Trials (Intro should be required reading for residents pg: 10-21)
https://www.icr.ac.uk/media/docs/default-source/default-document-library/pace_protocol_v12_clean.pdf?sfvrsn=130f3069_0NRG-GU005 - 3625 vs 70
https://www.nrgoncology.org/Clinical-Trials/Protocol/nrg-gu005?filter=nrg-gu005
Magnetic Resonance Imaging–Guided vs Computed Tomography–Guided Stereotactic Body Radiotherapy for Prostate CancerThe MIRAGE Randomized Clinical Trial
https://jamanetwork.com/journals/jamaoncology/fullarticle/2800541







Very well summarised!
I do not quite understand why you dont believe anything over low risk can have a 95% 5year BCF. Right dose, volume and ADT can achieve it regardless of fractionation (POP RT, WPRT arm)