Protons vs. Photons: Lung Cancer
A look at data for where toxicities might differ between the two modalities
Note: This Substack serves several purposes, one is simply for me to consolidate my own notes. This specific post relates to my work on another project in development but as it ended up being relatively thorough in the scope of the review, it is here as a reference document. Maybe it helps a few. This will be part of a broader series looking at acute toxicity data site by site.
The Backdrop:
What are the potential acute toxicity improvements within lung cancer treatment with a move to proton therapy?
To begin: This is a tumor site where we have randomized prospective data - in a passive scanning dominated trial.
Bayesian Adaptive Randomization Trial of Passive Scattering Proton Therapy and Intensity-Modulated Photon Radiotherapy for Locally Advanced Non-Small-Cell Lung Cancer
MD Anderson deserves credit for running one of the only trials attempting to answer this question published to date.
From a global perspective, as most will know, the trial was negative. It was published in 2017 and, if anything, the passive scatter proton therapy (PSPT) patients did slightly worse - IMRT had a 6.5% Gr3 toxicity rate and PSPT had a 10.5% Gr3 toxicity rate.
One can try to look for positives and find reasons for the lack of difference, but in simple terms, this trial using only passive scanning in the lung was negative for the primary objective of cumulative radiation pneumonitis.
So while passive scanning seemed to produce better dosimetric outcomes, it did not translate to a clinical benefit in this well designed study by experts in our field.
On the other side of the argument would be a retrospective series out of Penn arguing that there is a real reduction in Grade 3 acute toxicity:
Comparative Effectiveness of Proton vs Photon Therapy as Part of Concurrent Chemoradiotherapy for Locally Advanced Cancer
First off - clearly randomized prospective data is superior to anything retrospective, but I think this is interesting to at least consider that there might be some benefit - somewhere - maybe (if it isn’t all attributed to confounding differences).
The trial contained 132 lung cancer patients and 195 IMRT lung cancer patients - the study was much broader in scope looking at Grade 3 toxicities across patients receiving definitive radiation with concurrent chemotherapy for any site. But today we are looking at just lung toxicity. In the overall study, Grade 3 or higher toxicity in the 90 day window was 27.6% in the IMRT cohort vs. 11.5% in the proton cohort. I think, based on the randomized trial above, they looked at the lung cohort closer, but there is little said except for:
Exploratory subset analysis of the patients with non–small cell lung cancer in our cohort revealed a reduction in overall toxicity of at least grade 3 with proton therapy that was comparable to the reduction seen in the entire cohort.
So we have a negative trial designed to show pneumonitis differences and a retrospective series that shows a more general reduction in toxicity (Grade 3 - ER / hospital type toxicity) related to the treatment.
Prospective Patient-Reported Symptom Burden
The concept of lower toxicity is supported by a small longitudinal observation study out of MD Anderson. It includes patients treated with protons (n=26), IMRT (n=34), and 3D (n=22) approaches. Here is the take-home:
3D is worse than either IMRT or protons and protons reached statistically less toxicity compared to IMRT.
we found that pain, as a major esophagitis-related symptom, increased more during therapy (P=0.019) and decreased more after (P=0.013) therapy in the 3DCRT and IMRT groups than in the PBT group. Compared with the PBT group, the 3DCRT and IMRT groups reported greater decrease in systemic symptoms (fatigue, drowsiness, lack of appetite, disturbed sleep) after therapy (P=0.016).
So this trial argues for a potential decrease in esophagitis and then a less commonly studied single CTCAE toxicity of fatigue. The trial uses patient-reported symptom burdens and CTCAE toxicity is not presented nor was it studied.
The National Trial: RTOG 1308:
On the heels of the MD Anderson trial design and based on other retrospective data, a larger national lung cancer trial was developed. RTOG-1308. It is a randomized trial via the NRG and RTOG looking to compare image guided motion-managed proton radiation to image guided motion-managed photon radiation. It began accrual in 2014 (The Bayesian trial accrued from 2009 to 2014 FYI) and completed accrual in just September of 2023. So it took nearly a decade and the timeline is important as many patients in this trial will also have been treated with passive scanning approach - simply until about 2018, pencil beam was far less common.
Why bring this up if it isn’t published and we don’t have data. Because, this is a good starting point for references. Anything that has been through this level of review has a ton of input and the background and introduction SHOULD have the best of the best data referenced. (Good for residents to understand where to go and how do get things done quickly / efficiently).
So Step 1: What are even potential opportunities?
From my perspective, if there isn’t an improvement in dose to a structure, there is no argument for less toxicity. Protons aren’t magic - if they work, it is due to dosimetry improvements.
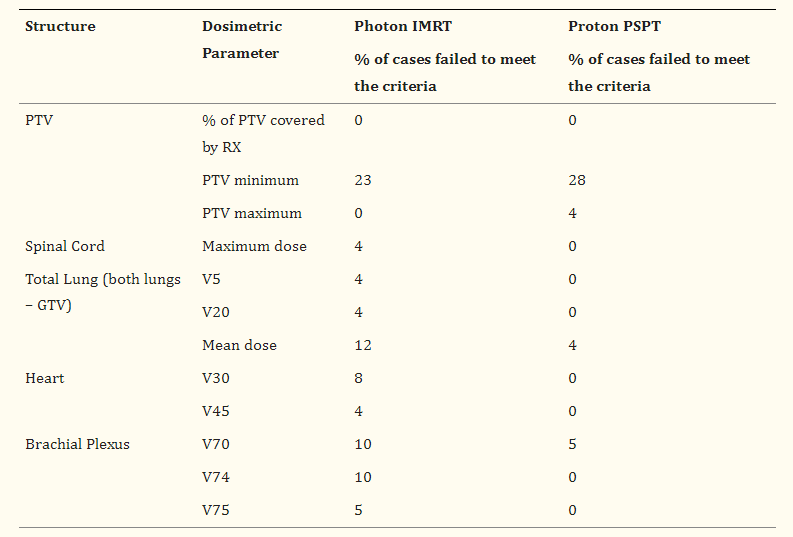
Interestingly, RTOG 1308 did a lot of pre-trial work looking at this opportunity question. It is summarized in the table below (Table 4 from this paper).
So from an acute toxicity, I personally would ague to disregard spinal cord and brachial plexus. Spinal cord will be safely constrained and we’d need 2 yrs to have any chance to see anything in the brachial plexus
That leaves us with small cohorts in the photon arm that do not meet total lung metrics (primarily mean dose with 12% of this cohort) and heart dose metrics related to 30 and 45Gy (heart dose at 60 and 66Gy were actually non-significantly worse in the passive scanning proton arm).
A note on esophageal dosimetry. As we discussed above, there is some hint of benefit in the MD Anderson data for improvement in pain due to less esophageal toxicity. This paper presents esophageal dosimetry expectations. They are shown below:
Lower dose benefits in the proton approach but with slightly worse performance in higher dose regions. Compare that to the heart figure below where, more simply, protons deliver less dose.
So from this work, I would argue we have two primary metrics: 1) lymphopenia due to integral dose and 2) radiation pneumonitis. (The third is dysphagia, but that appears more difficult based on dosimetry). Really, I think that is about it. And if you look at the randomized trial, the radiation pneumonitis data (certainly within passive scanning) has been refuted in prospective setting (see Figure 1 above).
As an aside, I think there IS opportunity within cardiac substructure dosing that is likely additive to the mean heart dose improvement cohort, but again, that is really a 2 year cardiac event metric and not an acute toxicity metric.
There is some hope: RTOG 0617.
Ironically part of the “hope” for protons using passive scanning approaches in the lung come from a trial that showed that more dose to the cancer resulted in worse outcomes. A must know trial for our field looking at dose escalation in lung cancer from 60 Gy to 74 Gy.
It found the higher dose arm lived significantly less. Overall survival was 28.7 months in the standard arm and 20.3 months in the dose escalation arm. The trial enrolled patients from 2007 to 2011 - approximately half were treated with 3D approaches / half with IMRT (so this is not directly compatible to IGRT / IMRT of today but likely shouldn’t be quickly dismissed either).
And beyond survival, it also showed significant lymphopenia risk in this cohort. 21% of these patients reported Grade 3 or higher acute lymphopenia which is likely related to low dose volumes (~3Gy can kill T-cells).
Because, as radiation oncologist, we believe in dose - a lot of work has been done trying to figure out what happened in this study. Learn and get better. Here are a few of the publications linking dosimetry to survival from this trial.
Demystifying the Results of RTOG 0617: Identification of Dose Sensitive Cardiac Subregions Associated With Overall Survival
Base of heart is important
Here is another:
Left Anterior Descending Coronary Artery Radiation Dose Association With All-Cause Mortality in NRG Oncology Trial RTOG 0617
LAD dose is important
And another, the long-term results from the trial:
Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non–Small-Cell Lung Cancer
Heart v5 is important
And a final one for us today:
Modeling the Impact of Cardiopulmonary Irradiation on Overall Survival in NRG Oncology Trial RTOG 0617
Modeling atria D45%, lung mean, pericardium and ventricle dosing (min to max % ratio) predicted overall survival.
(there are more in the literature, but making a point)
The point is:
Secondary analysis seemed to find quite a few options - all heart related that tend to link up overall survival to heart dosimetry metrics. The bad part is, that means we really don’t know understand the clear “best” metric even amongst these options. And these are from one trial with now out of date systemic therapy with many treated with 3D therapy.
And really, these are survival endpoints - not early acute toxicity. Sure the people that got sicker didn’t do as well but protons really didn’t change that hazard ratio.
Looking back, I must say, the odds seem stacked against RTOG 1308 for finding any improvement in acute toxicity beyond lymphopenia and hematologic (data to follow below). There will be some pencil beam patients and maybe we learned more and progressed more on the proton side than on the IMRT side in the last 10 yrs but after looking at the data broadly I honestly think that is doubtful.
Try two: Does Pencil Beam Offer Hope
Starting point - there is a lung PBS trial ongoing at Emory - single institution, single arm trial - it should have some decent data in the background. Right? Let’s go through the references.
Ok, a SEER dataset study and two dosimetry studies that look at 3D… we’ll move on.
There is the old MDACC Phase II trial - 74Gy - produced a mean OS of 29.4 months - for 2009 that is pretty good - granted - highly selected. (It was the pre-cursor data to the Bayesian trial - makes sense, easy to remember). Next, a similar trial from Japan giving 78.3 Gy back from 2001 to 2008. Median survival more than 2 yrs.
Both of these were back in the days of using proton therapy to PUSH doses rather than do the same dosing and trying to prove some slight improvement in QoL or a reduction in toxicity. Shame, in my opinion, that we lost that aspect over the decades. It is the centerpiece of the argument for the use case of protons in liver cancer today - with protons we use significantly higher doses.
But realistically, this trial doesn’t contain any real data that justifies anticipating less acute toxicity.
Recently there was strong data for lymphopenia. I review it in detail here:
Proton Therapy for Lung Cancer
My End of Year Review of the Proton Literature noted just how strong this article was placing it at number 2 on the “best of” list for 2023: Today, we’ll look a bit closer at this study and link up one additional publication that continue to show support for the idea of using protons in the treatment of lung cancer to decrease toxicity and improve outcom…
It is a prospectively collected series of consecutive patients were patients chosen for IMPT (intensity modulated proton therapy) via 4 criteria.
From the Supplemental data - the trial used Normal Tissue Complication Probabilities to parse a cohort of ~280 patients. The high risk patients were treated with protons. They were defined as high risk if they met one of the below criteria:
Reduction of grade ≥2 pneumonitis by 10%
Reduction of grade ≥2 esophagitis by 10%
Reduction of all-cause mortality at 2 years by at least 2%
Reduction in the sum of esophagitis and pneumonitis grade ≥2 by 15%
As an example, below is the predictive model for Grade 2 or higher esophagitis from RayStation RayBiology module. My point being, this type of straightforward predictive analysis is coming / is here today and can pretty quickly be integrated into your clinical decision trees. (I do NOT know which specific modeling approaches they utilized in this trial - I dug, but I could not find specifics. To me, what they used should become considered as standard since they were then validated in the study).
1 in 4 patients met those criteria. So from this trial, 1 in 4 lung cancer patients were anticipated to benefit to a measurable level with proton therapy.
It is interesting to note that once again, both radiation pneumonitis and esophagitis enter the calculation for who would benefit. I looked for specific numbers of how many qualified under each criteria - I did not find that information on my review.
A bit of math can help estimate the potential improvement. For example, I’d guess predicted risk for many in the IMRT arm for pneumonitis might be 6-8%. Not 0% but <10%. And in the proton arm likely was a bit above 10% - say 12%. I couldn’t find this data but those are likely reasonable estimates. From that, I assume this trial argues for about a 5%-7% reduction in Gr2 pneumonitis risk and a similar reduction in esophagitis. Honestly those are very small anticipated changes in Grade 2 toxicity.
These Grade 2 toxicities differences translated to a 20% reduction in Grade 3 lymphopenia - in what becomes a higher risk population.
In summary, that trial argues that acute toxicity reductions in Gr 2 esophagitis and pneumonitis (likely around 5%-7%) paired with lower heart dose (for the all-cause mortality), paired with integral dose reduction translate to rather large differences in lymphopenia when utilizing protons over IMRT in high risk populations.
So, in a real way, this is very similar to the Total Toxicity Burden approach on the MD Anderson Phase IIB esophageal trial - individual metrics for toxicity improvements are quite small, but when they all link up - you ultimately see an improvement in patient outcomes. In the esophageal data, it was shown via a total toxicity metric and here, it is shown in a dramatic improvement in lymphopenia.
This approach using normal tissue complication probabilities, to me, clearly supersedes the now older passive scanning Bayesian trial data. It is larger, more modern, multi-institutional and uses predictive risks to parse a lung cohort showing the HIGHER risk group treated with protons had 20% less Grade 3 lymphopenia than the lower risk group treated with IMRT. It is a bit disconcerting to me the prior data for both radiation pneumonitis and esophagitis is very limited, and in places, negative, but the criteria were chosen and did seem to “work” in finding a cohort to benefit from proton therapy.
Summary:
In summary, that is all I found. For nearly 30 years of clinical use. The data is quite sparse and not until late last year, could I find really strong data really supporting the concept for improvement. While one can argue against it by arguing a lack of supporting historical data paired with the older passive scanning data, I’m not sure that is wise as a long-term strategy.
Based on this work if you look at Gr3 or higher acute toxicity, there appears to be a very limited difference that one can anticipate beyond lymphopenia for the use of proton therapy. Toxicity data in the Baumann trial is not broken down from the information publicly available and I’m not sure a retrospective analysis like that should be parsed in that manner. I do think data supports that protons potentially CAN still be beneficial for OS via heart dosing but my review appears to point to lymphopenia or a total toxicity metric being required in order to find any real statistical power to see any difference in acute toxicity.
It should be obvious, I still find the trial out of the Netherlands and Italy to be really powerful. It argues that the highest risk ONE QUARTER of advanced lung cancer patients, likely benefit from a referral for proton therapy today. At the same time, the subtle differences in Gr2 toxicity argue that even if this is 100% correct, we still need to be very precise in the questions and answers we are asking or we will get answers that look like there are minimal to no differences.
Next up, we’ll look at the esophageal data - I do think it is quite possible that that data review will add additional content to this review but since each site holds a randomized data set, I wanted to keep them separate and not roll everything into one big post. If I missed a dataset or something, post below or reach out - happy to add to have this be more comprehensive.
As always, thanks for following along - We’re in an amazing field of medicine offering incredible value to our patients. Keep fighting for better.










I truly believe that Proton treatment IMPT would be less toxic to the heart, lungs and esophagus for Stage III b ( bilateral MN or Hilar nodes) and very large unresectable Stage III A patients compared to IMRT.
We need to wait for the results of NRG 1308.