Prostate Cancer: SBRT Journey Part 3: Questions, Outcomes, Trials, and New Metrics
Today, a deeper look into areas where I think our data can be strengthened.
In Prostate Cancer: SBRT Journey Part 2: Intermediate Risk Outcome Data, we looked at the published outcome data - focusing on intermediate risk outcomes following SBRT approaches. I think as you look closer, most would agree that - at a minimum - the data is less consistent than for traditional or hypofractionated approaches. And that inconsistency leads to a rather wide range of expectations for what is a very common risk category to treat with radiation.
Today, as promised, we’ll go a step deeper into the weeds and look at some of my personal questions in the literature beyond simply, the outcome data. Again these stem from my personal clinic experience where my post-treatment PSA kinetics appear inferior with SBRT vs. 7250 up as median follow-up is now beyond 15 months.
This post will look at 4 additional questions: areas where I would like to have stronger / better answers in our own data (we discussed outcome data in Part 2). We’ll then look at 3 issues with outcome reporting that clearly have potential to introduce a level of variance and bias into our published literature. And then to finish up, I’ll propose ways in which we can address or minimize these issues as we move forward. (that is a primary goal of this Substack - not just to ask questions, but to help propose ways in which we can be better moving forward) That is a brief summary of where we’ll go.
Questions:
EQD2 Calculations:
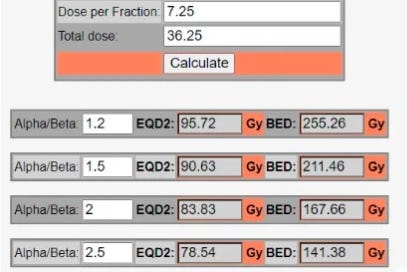
We use these calculations frequently today and we use them across a variety of doses and fractionation schedules, but we also need great clinical data to verify these concepts - especially as we move towards SBRT. For prostate cancer, the exact alpha/beta tends to have far greater impact on your EQD2 - it simply matters much less in a hypofractionated setting. Here are some examples:
As shown, small differences in the alpha/beta have FAR larger impact in SBRT - from 2.5 to 1.2 hypofractionation will move ~4%. Compare that to a 23% difference when looking at 40 / 5. Alpha/beta ratios continue to be quite varied in our literature today. For example the large meta-analysis performed in the US used 2.5 for its dose regression analysis. Meanwhile Netherlands used 1.2 for the FLAME trial. PACE background discussion tends to be under 2. These differences alone represents a 22% difference in EQD2 dose for 36.25 Gy. (95.72 at 1.2 vs. 78.54 at 2.5).
And the larger question of whether this very simplistic calculation applies as well in SBRT dosing I believe is still largely unanswered. Most of the data in SBRT I found was for lung cancer. In prostate cancer, where alpha/beta ratios are far lower, I think we have less certainty today. We have great data in support using this simplistic approach in hypofractionation, but less I can find in SBRT. (if you know of really strong datasets - please leave a comment).
PACE B and NRG GU005 will be critical datasets to begin to address this specific question in prostate cancer and help to refine this piece of data. They both use 3625 / 5 and both compare to standard dose arms that are ~78-80 Gy. At 2.5, these are all equivalent doses - as it moves to 1.5 or 1.2 SBRT becomes higher dose with a difference greater than 5%. Historically we’ve able to pick up about 5% or more dose difference in these prostate trials - think of CHIPP where 57 was not equal to 60 - so these should help define this issue.
Toxicity:
Between traditional fractionation and hypofractionation, I didn’t see much change - maybe a little but really pretty subtle. But jumping to SBRT, I quickly looked to adjust my off-trial patients. I made two adjustments. I adjusted block margins tighter by 1mm to 1.5mm and made scheduling adjustments - so two clear changes to my approach based on clinical toxicity. To me, it was appreciably different.
What type of difference was I seeing? The old rule was differences need to be at least 5% more to judge clinically. In my 20 yrs of practice, I think this is a ballpark correct estimate. Here, I thought it was at least that different. And I think you see it subtly in the literature - I saw this table recently referenced to demonstrate low bowel toxicity in SBRT. (ref 1)
The paper containing this table reads very favorably - transient effects - good recovery to baseline. The figures / curves look great as well. But on closer look - I don’t believe I see this in my clinic at 7250 in 29. Moderate problems in 10% with urgency / frequency and 20.5% with very small-small or moderate-big bowel INCONTINENCE issues.
Simply stated, this is a larger bowel impact than I believe I see - and for the last month I’ve been asking my patients about this in far more detail. Bowel toxicity isn’t zero but it is far less than this data which reports 50% (39+11) with bowel urgency and 20% (17+3) with incontinence issues. (note, clarification: there is a “baseline” toxicity level that I should have probably excluded from 50 and 20 - making them 28 and 15 to be more equitable on the findings).
In fact, the vast majority of issues I see relate to men with a hip replacement or pelvis treatment. Again, I appreciated the difference in just a dozen or so cases with the shift to SBRT. It was more than what I read, and I believe what I see.
Is it manageable? Yes. Are we closer to, as I say, the razor’s edge? I believe yes.
And I’ll point back to PACE B as well (ref 2, 3). Here again I see some divergence between the headlines and the details in the paper.
Interpretation (abstract conclusion)
In the PACE-B trial, 2-year RTOG toxicity rates were similar for five fraction SBRT and conventional schedules of radiotherapy. Prostate SBRT was found to be safe and associated with low rates of side-effects.
But if you read the paper, you’ll notice a secondary endpoint of the trial was cumulative Gr2 toxicity. Here you see a different answer than what is in the abstract.
At 2 years, the cumulative incidence rates of RTOG grade 2 or worse genitourinary toxicity were 10·6% (95% CI 8·0–14·0; 45 events) for control radiotherapy and 18·3% (14·9–22·4; 75 events) for SBRT (hazard ratio [HR] 1·80 [95% CI 1·25–2·61]; log-rank p=0·0015). The corresponding figures for CTCAE grade 2 or worse genitourinary cumulative toxicity were 19·8% (95% CI 16·3–23·9; 84 events) for control radiotherapy and 32·3% (28·0–37·0; 132 events) for SBRT (HR 1·73 [95% CI 1·32–2·28]; log-rank p=0·0001).
Those are highly significant differences in cumulative acute toxicity nearly doubling the cumulative rate of Gr2 toxicity. (I really dislike abstract tone not matching well with detailed results - on Substack in editorials, maybe - in our medical literature, report / document findings that are clearly data supported in the abstract).
I think what I see in clinic is consistent with difference in cumulative toxicity.
And then look at this recent publication: The Association between Acute and Late Genitourinary and Gastrointestinal Toxicities: An Analysis of the PACE B Study (ref 4). It looks at the PACE-B study and finds that on multi-variate analysis, acute toxicity is associated with late toxicity (both for bladder and bowel toxicity) - they sliced and diced the definition of “late toxicity” various ways and regardless: more acute toxicity » more late toxicity. p<0.0001.
So… if you see more acute toxicity, you will see more late toxicity. Makes sense - easy to remember - oh yea, and importantly, now represented in randomized prospective data for SBRT and IMRT for prostate cancer. To me, that argues against the “transient statements” that all is well after the acute toxicity resolves. These statements are very common in our literature, but would appear to be contradicted in this broader randomized prospective setting.
And finally then pair that with the recent Accelerator podcast where PACE is looking to de-escalate to reduce toxicity - dropping whole prostate dose to 30/5 for uninvolved regions - a ~30-40% reduction in EQD2 dose. I would assume they are seeing REAL toxicity to make such an adjustment. (and as we discussed just one paragraph above, acute toxicity is translating into late toxicity data).
Realistically, I’m not sure I see anything in my clinic that any dose reduction could / would fix at 7250 / 29 in my current practice. I did the toxicity publication for the original MDACC dose escalation trial (ref 5) and I remember thinking that below certain cutoffs - you just begin to see “noise”. In other words, almost a placebo rate of rectal bleeding or nocturia or frequency - low rate, very mild and really nothing that reducing dose would affect - more age related things that just happen. I think that is where I am in my current hypofractionated approach.
In fact at this time, I’m much more concerned about limiting ADT than treatment toxicities. For some UIR cases, I’ve actually increased my margin around clear MRI disease to 5mm in all directions and tweaked dose another ~2% higher (based on FLAME) because I see so little toxicity it seems like an obvious thing to do. More reliance on the radiation and less reliance on ADT. Again similar to the PSA kinetics we discussed in Part 2, my toxicity outcomes were just not quite consistent with what I expected in my clinical experience.
And then my kinetics data, VERIFIED:
As we reviewed in the prior post, my kinetics are different between my different treatment fractionation approaches. And yep, I trust what I see in my clinic. And if you look in the literature, it appears to have been replicated at Fox Chase, Early PSA kinetics for low- and intermediate-risk prostate cancer treated with definitive radiation therapy (ref 6).
This series was just published a few months ago - end of 2022. 1047 men treated with a variety of approaches and they looked at kinetics. It is the only series I found similar to the database I keep. Similar to my experience, the SBRT cohort is small - they include just 52 patients. And similar to my database follow-up is short and they report findings for the two-year follow-up period. Their SBRT cohort was treated at 37 Gy / 5 fx - so it is cooler than my schedule - but likely still around 90 Gy EQD2.
But they also found something I see. They reported it a bit differently but over 50% of traditional patients treated to 78-80 at 2 Gy or 70.2 at 2.7 nadired under 0.5. In contrast only 23% of SBRT patients nadired under 0.5. (this is 2 yr kinetic data and not long-term PSA data).
The paper tries to argue that kinetics are slower to fall but will likely catch up with more time. In the discussion they point to two other references:
Below, I include a paragraph directly from the Fox Chase paper discussion because I think it is quite interesting to read their appraisal:
While we did not anticipate the latent PSA response to ablative therapies, these findings are not inconsistent with other studies. Kishan et al. compared SbRT, HDR, and CFRT and reported significantly longer times to nPSA using ablative techniques when compared to CFRT. The proportion of patients reaching nPSA ≤0.5 and the median time to nadir were 76% and 35 months for SbRT, 76% and 33 months for HDR, and 45% and 21 months for CFRT, respectively (1). Levin-Epstein et al. recently published a large multi-institutional analysis of PSA kinetics for patients treated with SbRT, HDR and LDR. Patients treated with LDR were reported to have significantly lower nPSA levels, with 72% of the cohort reaching an nPSA ≤0.2 and a median time to nPSA of 51 months. Although SbRT and HDR cohorts had median times to nPSA of 44- and 37- months, only 48% and 56%, respectively, reached the same threshold of nPSA ≤0.2 (2).
My comments regarding this assessment: In reference paper 1, SBRT nadirs lower with larger decay rates - yes CFRT reaches its nadir earlier but the nadir it reaches isn’t nearly as low - to me, this assessment is likely conflating two issues. I see it as PSAs don’t continue to decrease and therefore reach some marginally higher nadir value earlier - I don’t think that is “good” - in fact quite the opposite. In paper 2, it might be as simple as LDR is better than SBRT (it is more dose after all) - again the lowest of the low values takes longer to reach. I don’t think either of these papers strongly argues that at a given time point, higher is better which is what I see in my series and what the 1000 patient Fox Chase series sees.
I don’t know what is going on here or in my series for sure - I believe both myself and the Fox Chase team did not expect to see our own data. But if you look back at the Ma 2000+ series (ref 9), they get pretty low / pretty fast. Clearly more than half in that series get below 0.5 as the mean I calculated was 0.37 at the 2 yr mark - that is a low early mark. Something is not consistent. That large multi-institutional series seems to “work”, whereas I have questions about my series and the Fox Chase series.
Maybe it will be that this Fox Chase series and my series will both flip and, in the long run, the SBRT groups will outperform beyond 2 yrs. But I doubt it. I haven’t found that in any external data or in implant data and I can’t find an example in SBRT data.
My gut says there is nuance to the approach or patient selection and somehow, something isn’t quite as easy to translate broadly and / or with protons in particular, there is some additional LET / BED interaction for low alpha / beta particles that is poorly understood.
After digging through the weeds, I still think for PSA kinetics:
Lower is Better and Faster to Lower is Better
Finally: Gossip (haha)
Yep. I have talked to a few people and they have seen the same thing I think I see: that post SBRT nadirs aren’t as low - especially in the first 2 yrs or so following treatment. PSAs don’t fall as fast and they cause more concern for the patients and the physicians. I’m purposefully not naming sources: so the old “anonymous sources” debate. You can believe my story here or choose not to. But know that I have more freedom than most to go against the grain.
So I don’t believe it is just me. It is there in the Fox Chase data and it exists in other datasets, at least in quite corners of our field far from what is highlighted our social media posts it seems to persist.
OUTCOMES:
One would think outcomes are pretty much binary - yes or no. Fail or be cured, but there are multiple additional factors that influence reported outcomes in prostate cancer and have significant potential to introduce bias.
Follow-up Duration:
Phoenix definition was developed nearly 20 yrs ago. It has held up very well. It has known issues and the developers knew of these issues. The original document requires a reporting length no longer than 2yrs less than the median follow-up. Here is the quote from the reference document.
To avoid the artifacts resulting from short follow-up, the reported date of control should be listed as 2 years short of the median follow-up. For example, if the median follow-up is 5 years, control rates at 3 years should be cited.
Yet look at what we do today. Median follow-up in the 6000 patient meta-analysis was 39 months, yet the conclusions are published at 5 years and 7 years (ref 11). Per Phoenix, it should be no more than 15 month data. (I did NOT roll back through the 10 trials and specifically look at Table 1 median and re-calculate a median follow-up - I took the abstract number - but I bet the 2000+ patients aren’t followed for a median of 9 years to report 7 yr data.)
Phoenix describes a pattern of PSAs and therefore know we need data - years of it - for it be accurate, yet our standards in our modern medical literature are simply gone.
And you just need to look to the Fuller data (ref 12). Its a series that has been updated and it demonstrates this issue directly.
The first publication - the one included in the meta-analysis - puts bRFS for intermediate disease at 92%. But in the “10 yr” publication (median follow-up of 5.5 yrs), the Kaplan-Meier curve showed ~92% and ~84% 5 yr bRFS for FIR and UIR respectively (me taking data from curve so approximate). A fall to clearly <90%. And the abstract reports 10 yr outcomes declining to 84.3% and 68.4% respectively.
(Remember per Phoenix definition, median follow-up should be >7yrs to report 5 yr results so the “real” 5 yr result should be ~88% (between 92% and 84%) for FIR and ~76% (between 84% and 68%) for UIR. - quite a distance a from the 92% reported in publication 1.)
The point here is simple: time matters - especially with Phoenix. Early reporting of “long-term” outcomes introduces significant upward bias and it happens nearly everywhere today.
Biochemical disease free survival vs. disease free survival:
Today, publications often report bRFS - look again to the meta where Lee trial (ref 14) can be represented in two very different ways. First take the presented approach for the Lee data where SBRT for relatively high risk cohort achieves 89.7%. With 13% low risk, 58% intermediate risk, and 29% high risk - this study represents one of the largest cohorts of high risk disease in the entire meta-analysis.
They report exactly what they say they report: bRFS. But if you go track down the reference and just read the abstract, you get a different impression. In the abstract of the actual Lee paper, they note that progression free survival was 71%.
Left is biochemical and right is progression free - Figure 1 from the Lee publication. If I were a patient, I’d count bone metastasis or ADT along with the failures. And I hate to even point out, median follow-up is 63 months, so expect these to fall with more follow-up.
(this is why “numbers of patients at risk” along the x-axis are important on these curves - it helps us evaluate the data knowing how many patients remain at a given time - if you write papers, include them please).
In contrast, the Widmark data (ref 15) uses disease free survival. No caveats, no reason to dig through and find exceptions. One is good, the other, in my opinion, is far less.
I’ll keep things simple here. I don’t like this shift. I guess I’m old. If I get treated for lung cancer and I develop brain mets, I’d assume that shows up in the data regarding the question: “was treatment successful?” but apparently in prostate cancer, this is not routinely the case.
When do YOU scan?
PSMA scan are in the community. When you scan will be important. My guess is this scan can and will dominate failure patterns in years 2-5 for many men following treatment. I can see two groups of physicians - ones where someone has financial gain to order the scan or create “downstream revenue” and ones where management is far more conservative (broadly think of US vs UK). This is will be a major factor in comparing US data to data outside of the US. I’m assuming the non-US data should have less PSMA scan usage and more reliance on PSA - just look at the dramatic difference in the recent observation UK PROTECT trial (ref 16) vs. NCCN Guidelines recommendations in the US - they are literally an ocean apart as I discussed earlier.
As of today, I don’t know of and haven’t seen recommendations on how this large variable that will affect outcome metrics should be integrated.
These three factors: duration, bDFS vs DFS, and when you scan patients can all dramatically impact reported control rates.
Trials and Kinetics
So I believe we’ll need two approaches - 1) trials and 2) kinetics.
Trials: NRG-GU005 and PACE B
Both of these trials are, overall, very strong pragmatic randomized prospective datasets. They will both be critical to our understanding of SBRT.
By design they counter reporting time, questions of bRFS vs. RFS and to a lesser degree, timing of PSMA scans. Prospective trials are the central data piece.
First we’ll look at NRG-GU005 (ref 17). It will report primary outcome measures of GI/GU toxicity and disease free survival at 2 yrs. There is NO way there will be any difference in disease free survival at 2 yrs - zero - at 5 or 7 yrs - perhaps. So realistically this is a toxicity trial - with a margin difference between the two arms - in fact, that is basically how it is presented in the trial description. In the traditional arm, margins are 8mm and 5mm and in the SBRT arm margins are 5mm and 3mm - that difference is LARGER than between the two arms of the MIRAGE Trial.
As a reminder, the difference in the MIRAGE trial was 2mm between treatment arms. In the MIRARGE trial, this margin reduction resulted in both GI and GU toxicity being markedly reduced with highly significant p values <0.01. So we KNOW that tighter margins create less toxicity. And meanwhile the risk for reducing margins is decreased long-term cure rates, but the primary 2 yr objective almost certainly negates that risk to zero.
In a way, NRG GU005 is therefore asking which of the following is correct?
The shift to SBRT offsets an improvement in toxicity due to lessening margins - ie toxicity is about the same between the arms.
Shortening the course, even with tighter margins is just worse - ie SBRT has greater toxicity despite having a proven known benefit in margin reduction.
Toxicity is reduced with SBRT - ie the benefit of margin reduction remains despite shortening the course.
In a very direct way, if SBRT “wins” on toxicity, it has shown a margin difference is NOT largely offset by fraction reduction. Which approach actually has toxicity with equal margins is not answered here, but rather in PACE B.
Overall it is a strong trial but it will be interesting to see how it is presented. I anticipate it will be described quite differently from how I just described the trial structure. At least now, you have heard both sides to make your own judgement on Day 1.
PACE B: A strong pragmatic trial looking at toxicity and disease free survival (ref 18). Straightforward, robust patient numbers - a balance of both Cyberknife and Linac treatments. 2yr toxicity results have been presented and in general, both arms showed rather minimal toxicity - not zero, but both appear well tolerated. As I pointed out previously, there is markedly more cumulative acute toxicity in the SBRT arm which as we discussed above translates to late toxicity.
Just a reminder, here are EQD2 comparisons for the three main dose arms in these two trials not at 2 Gy.
If equivalent, then the Alpha / Beta should be 2 or greater OR we need to tweak this model to better adjust for quick overall time and larger fraction sizes.
Both are excellent trials that should certainly help us move forward with our trust and understanding of expectations with SBRT for many men with intermediate risk prostate cancer.
And Kinetics:
Why kinetics?
Because kinetics minimize effects of early reporting of long-term results.
When comparing cohorts kinetics minimize those that avoid issues with disregarding clinical outcomes.
Kinetics minimize the impact of when PSMA scans are performed.
Kinetics can be more simply reported at earlier 1 yr and 2 yr timepoints.
Kinetics don’t fix everything, but I think they move our reporting in the correct direction - away from potential bias. At least, from my perspective.
And with that, we’ve gone into the weeds. In summary, we’ve addressed the intermediate risk data, EQD2 questions, toxicity data, publications showing slower PSA response, “my anonymous source gossip” regarding others seeing slower responses, as well as issues related to shortened follow-up in publications, lack of clinical progression in reporting of control, and touched on the impact that PSMA scanning will have on future publications.
Finally, as we look forward, I tried to simply argue we need both good trials and a return to kinetics to help solidify our best treatment options. I’ll spend a bit more time on explaining my stance on kinetics in the next article. I need a bit of time to walk through examples.
REFERENCES:
Proctitis following stereotactic body radiation therapy for prostate cancer
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4272823/PACE Trials (Intro should be required reading for residents pg: 10-21)
https://www.icr.ac.uk/media/docs/default-source/default-document-library/pace_protocol_v12_clean.pdf?sfvrsn=130f3069_0Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(22)00517-4/fulltextThe Association between Acute and Late Genitourinary and Gastrointestinal Toxicities: An Analysis of the PACE B Study
https://www.mdpi.com/2072-6694/15/4/1288Complications from radiotherapy dose escalation in prostate cancer: preliminary results of a randomized trial
https://pubmed.ncbi.nlm.nih.gov/11020558/Early PSA kinetics for low- and intermediate-risk prostate cancer treated with definitive radiation therapy
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9756964/SBRT and HDR brachytherapy produce lower PSA nadirs and different PSA decay patterns than conventionally fractionated IMRT in patients with low- or intermediate-risk prostate cancer
https://pubmed.ncbi.nlm.nih.gov/26850649/Prostate-specific antigen kinetics and biochemical control following stereotactic body radiation therapy, high dose rate brachytherapy, and low dose rate brachytherapy: A multi-institutional analysis of 3502 patients
https://pubmed.ncbi.nlm.nih.gov/32663537/Refining the definition of biochemical failure in the era of stereotactic body radiation therapy for prostate cancer: The Phoenix definition and beyond
https://pubmed.ncbi.nlm.nih.gov/34774650/Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference
https://pubmed.ncbi.nlm.nih.gov/16798415/Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies
https://pubmed.ncbi.nlm.nih.gov/30959121/Virtual HDR CyberKnife SBRT for Localized Prostatic Carcinoma: 5-Year Disease-Free Survival and Toxicity Observations - Fuller
DOI: 10.3389/fonc.2014.00321High Dose “HDR-Like” Prostate SBRT: PSA 10-Year Results From a Mature, Multi-Institutional Clinical Trial
https://www.frontiersin.org/articles/10.3389/fonc.2022.935310/fullStereotactic body radiation therapy for prostate cancer patients with old age or medical comorbidity: a 5-year follow-up of an investigational study - Lee SW
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4603131/Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial - Widmark
DOI: 10.1016/S0140-6736(19)31131-6Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer
https://www.nejm.org/doi/full/10.1056/NEJMoa2214122NRG-GU005 - 3625 vs 70
https://www.nrgoncology.org/Clinical-Trials/Protocol/nrg-gu005?filter=nrg-gu005
PACE Trials (Intro should be required reading for residents pg: 10-21)
https://www.icr.ac.uk/media/docs/default-source/default-document-library/pace_protocol_v12_clean.pdf?sfvrsn=130f3069_0www.protons101.com, home of the original Protons 101 website.
Content for the Protons101 blog written by Mark Storey MD.







Wow! so much information here. Love your anti "transient" POV. Watch out for Acute toxicity of 68/25 vs 36.25/5 from PRIME trial (HR/VHR) at ESTRO 23!
Really enjoyed reading this series of posts. I might have missed this, but were you delivering your SBRT with protons?