Protons 101: What happened to rectal spacer benefit in the MRI MIRAGE Trial?
Past "studies" say it works. This one maybe says not so much.
As always - author of one. If you see an error or omission, write or comment. I’d much rather this be a glowing review of rectal spacer data. But, the goal here is to call it like I see it.
DOES RECTAL SPACING REALLY CHANGE OUTCOMES?
As discussed in my personal covid story I’m back to reading literature more critically or maybe more cynically. Today we’ll look at rectal spacer data based on a table buried in the supplemental data of the MIRAGE MRI Trial.
To be clear, I have used a rectal spacing product for about 4 years in the clinic where I work - center wide I’d guess at least 1200 cases. The center was using it when I arrived and I continued the existing program with very few changes. I’ve thought it holds some benefit. I place it in most cases. At our facility, if we treat with protons, we MUST place fiducials (for IGRT) so it literally becomes one extra needle stick (to me that is different risk / benefit than if fiducials were not required). Dosimetry and planning metrics are easier to meet and broadly I believe that dose to normal structures should equate to toxicity.
But I’ll also say this after reading the data referenced on the Boston Scientific webpage. Do you ever feel you’ve been suckered? Maybe not completely but at least partially? Or maybe you paid $7 for the world’s best coffee and it was honestly just really bad and not worth $2. Well... Today kind of feels like that after spending a day in the weeds of the data on rectal spacing.
Rectal spacing is big business. For scale, Boston Scientific paid $500 million upfront cash with $100 million for sales-based milestones in 2018 for Augmenix. Since then it has been a MAJOR sponsor at ASTRO - plastered ads everywhere. It costs around $3500-$4000 (ref 1,2) (maybe slightly less today). To give you an idea, under the RO-APM, professional services are reimbursed $3260 for 90-days of physician care. So, ballpark, the gel is slightly MORE VALUABLE than the physician in the treatment of prostate cancer. So.. Let’s look at the data and see if you think the data is as good as having a doctor.
First the MRI MIRAGE TRIAL data:
Why MIRAGE data? It is high quality with good design in a prospective fashion. That makes it a better dataset than almost anything else.
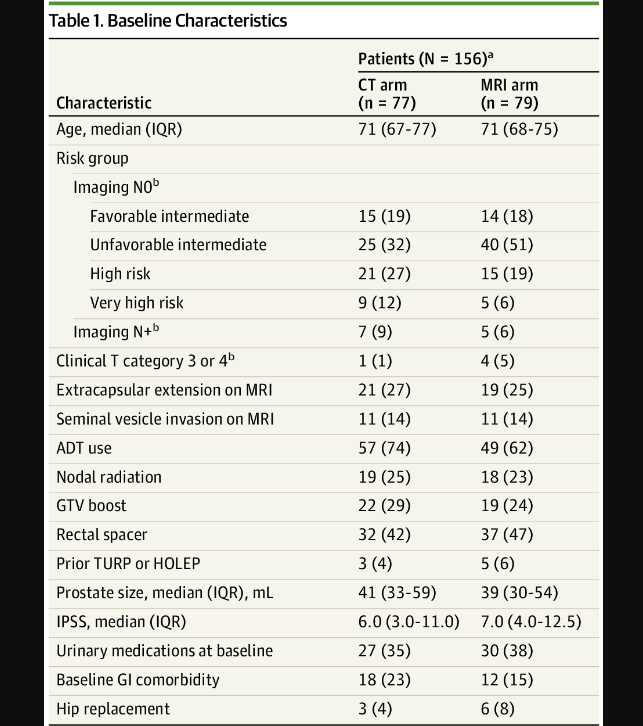
Here is Table 1 from the MRI MIRAGE trial - a prostate cancer trial looking at MRI vs CT treatment for prostate cancer. Technically, it is more nuanced than that, and I discuss it at much further length here.
First off - the point of the MIRAGE trial was NOT to evaluate rectal spacer but, ironically, I think it makes one think. If you notice below about one half of the patients in both arms had a rectal spacer placed. Total numbers would be 69 with Rectal spacer and 87 without - equally distributed by modality.
There are two main ones on the market today - SpaceOAR by Boston Scientific and Barrigel. They are very similar - not identical and I’ll likely do an article in the future with my personal experience comparing the two. I don’t know which was used - likely SpaceOAR based on market share but do not know and not sure it matters.
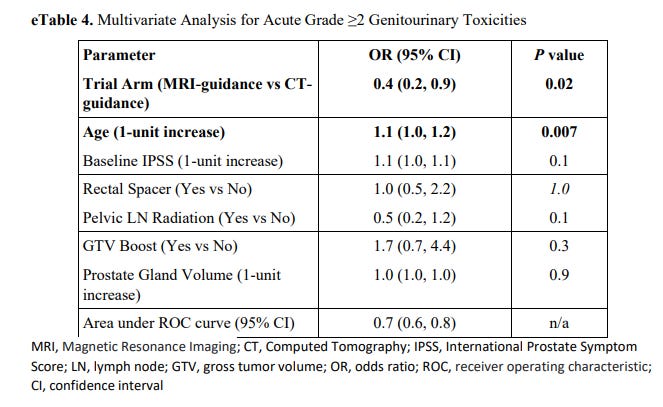
Why is this interesting to note? Because rectal spacer made no difference in outcomes with respect to bladder toxicity as shown below - zero.
This same table is not published for GI toxicity and that is a shame. The idea of a rectal spacer is stronger in GI toxicity so that table would be one step stronger to make this argument, but it isn’t provided from what I can find. (per Kishan, due to lack of events).
In summary, this prospective randomized trial does not demonstrate any difference in GU toxicity with a rectal spacer. Again, it isn’t designed to measure that outcome, but I’ll give an analogy.
If we were looking at stage and outcomes in a toxicity oriented trial - it would be a major flaw if stage didn’t correlate with control. We know that staging works. Here we have prior data showing rectal gel works but then here that feature is not replicated. If reductions are really 60%, as advertised, then one would think that difference would show up on some level here. Right?
But the MIRAGE trial shows zero impact on bladder toxicity. Contrast that with the SpaceOAR webpage graphics where there are many well put together very convincing graphics.
Example 1:
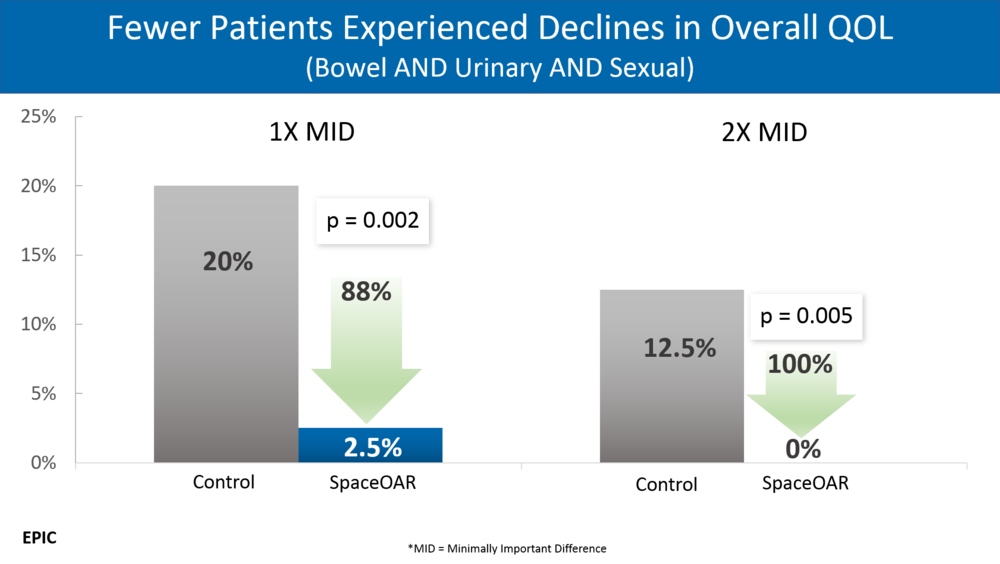
Example 2: (super impressive p values - a great clear graphic)
So at this point, I think it is very appropriate to compare the original trial and the original data’s strength to the data from the MRI MIRAGE trial. Because at least on some level, the results are not consistent. So let’s jump into the rabbit hole.
“The Three References” for SpaceOAR per the official site.
(ref 6,7,8). I use quotes because, although there are 3 references, they are all the same 222 patients. One randomized prospective multicenter pivot trial and then two additional secondary analysis.
Other data: There is a 2020 comprehensive review ( ref 9) on rectal spacers. Note: it does not utilize actual patient data. Of the 6 cohort studies, I recommend the Deutschland (ref 10) data as it potentially supports the concept for a larger difference in late toxicity over less differences in acute GI toxicity.
I agree with website focusing on the single randomized prospective pivot trial.
Kudos to the trial teams on the original study, seriously. The original study is well structured with a clear study design. Developing this work to prove technology advances is critical to our field. Well done on the study design and execution. But in science, we must look critically at the data and constantly reassess. So we dig and point out weaknesses, and in my opinion - those lie within the analysis and write up. Below we walk through what I see.
Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial
The original publication was released in April of 2015. Here is the original randomized trial design. There are two prospective randomized outcomes. Rectal v70 volume and Gr1 or greater rectal toxicity in the first 6 months. 221 patients enrolled. 2:1 in favor of rectal spacing.
Primary Endpoint Outcomes:
Rectal spacing did decrease the volume of rectum treated to 70 Gy. 11.7% » 3.3% with a highly significant p value of <0.0001.
The second primary outcome and only clinical primary outcome was negative. No difference in the number of Gr1 rectal toxicity in the first 6 months (p=0.7). Spacer at 34.2% and control at 31.5%. (I typed the order correct with spacing slightly higher) Wait? What?
(Honestly, I didn’t know this. I wish I knew everything all the time, but I don’t, ask my wife). This trial - as designed - ONLY shows dosimetry differences with no difference in the safety / clinical outcome. If you think about it, this is similar to the lung RCT for protons, which ah… wasn’t received broadly as a “win” for protons. That trial showed less heart dose but failed on providing a clinical outcome difference.
Realistically, I think you can make an argument that we should stop here.
Arguably, we now have two prospective datasets and any analysis of the original trial beyond the original intent of the trial is no stronger than the data from the MIRAGE trial. This prospective randomized controlled pivot trial was negative for the clinical endpoint. This same trial then has a secondary analysis on previously unspecified endpoints that appears positive (albeit from within negative primary trial) and now the MIRAGE dataset that, at least on GU toxicity, shows no benefit. Based on this, the most likely scientific “truth” in my opinion would be - no difference - or very little.
But we, as a subspecialty, often use it. So below is the data that makes the argument for the graphics.
Deeper into the rabbit hole we go.
The original paper continues well beyond the primary endpoints in what I would say is largely an exploratory analysis. I’ll give some benefit of doubt and assume the QoL data was perhaps a secondary planned endpoint, but the paper doesn’t really state that as fact (and I can’t find the pre-release trial info). I don’t think the remaining analysis is useless. I just think it is important to understand context - it is a secondary endpoint analysis inside a trial where the primary, clearly defined primary endpoint, failed.
Here is a quote that honestly disappoints in the “Results:”
At 6, 12, and 15 months, a lower proportion of spacer patients reported declines in bowel QOL relative to those of control, with 11.6% and 21.4% of spacer and control patients, respectively, experiencing 10-point declines at 15 months.
It is then followed by a p value of 0.087 which would then make an accurate assessment of the above as something like
(My version) At 6, 12, and 15 months, there was a possible trend towards a lower proportion of spacer patients reporting declines in bowel QOL relative to those of control, with 11.6% and 21.4% of spacer and control patients, respectively, experiencing 10-point declines at 15 months but it was statistically not significant.
Unfortunately, this is not the only example that appears to be biased. Some are subtle like this one from the abstract.
There was no late rectal toxicity greater than grade 1 in the spacer group.
This IS true, but look closer. From the results, there was only a single case of Gr2 or higher toxicity in either arm. 1 Gr3 toxicity and no Gr2 toxicities. Here is the more detailed statement regarding trial toxicities.
Late rectal toxicity (3-15 months) was observed in 2.0% of the spacer patients (1 grade 1 rectal bleeding, 1 grade 1 rectal urgency, and 1 grade 1 proctitis) and 7.0% of the control patients (3 grade 1 rectal bleeding, 1 grade 1 rectal urgency, and 1 grade 3 proctitis). The spacer group reduction in late rectal toxicity severity was statistically significant (P=.044), with no spacer patients experiencing grade >1 late rectal toxicity.
Statistics can say anything. They can be useful. They can be a distraction. In this case, if the ONE Gr3 complication occurred in the rectal spacing arm, TWO sentences from the abstract results would be invalidated. So while it may be true, just remember that context when assessing the strength of this secondary endpoint analysis.
As we’ve reviewed, the primary clinical endpoint is negative. The trial clearly demonstrates a dosimetry benefit. But here is the abstract which seems to give a very different impression. The BOLD ITALICs are mine with comments to these bolded statements below.
Results: Spacer application was rated as "easy" or "very easy" 98.7% of the time (1), with a 99% hydrogel placement success rate. Perirectal spaces were 12.6 ± 3.9 mm and 1.6 ± 2.0 mm in the spacer and control groups, respectively. There were no device-related adverse events, rectal perforations, serious bleeding, or infections within either group. Pre-to postspacer plans had a significant reduction in mean rectal V70 (12.4% to 3.3%, P<.0001). Overall acute rectal adverse event rates were similar between groups, with fewer spacer patients experiencing rectal pain (P=.02).(2) A significant reduction in late (3-15 months) rectal toxicity severity in the spacer group was observed (P=.04), with a 2.0% and 7.0% late rectal toxicity incidence in the spacer and control groups, respectively.(3) There was no late rectal toxicity greater than grade 1 in the spacer group. (4) At 15 months 11.6% and 21.4% of spacer and control patients, respectively, experienced 10-point declines in bowel quality of life.(5) MRI scans at 12 months verified spacer absorption.
Conclusions: Spacer application was well tolerated. Increased perirectal space reduced rectal irradiation, reduced rectal toxicity severity (6), and decreased rates of patients experiencing declines in bowel quality of life. The spacer appears to be an effective tool, potentially enabling advanced prostate RT protocols.
My Comments:
1: Sure - I’m not going to be the “one” who says it was “hard”. The procedure is straightforward. It is not without risks - both complications and placement risks.
2: Misleading at best - From the results, it is more accurate to state that if you exclude the acute toxicity from the procedure, they were able to find (on further unplanned analysis) that the gel patients appeared to have less specific pain (1 of 11 CTCAE Rectal toxicities) during the course of radiation. (Another way of looking at it would be that it argues that the procedure has enough toxicity to balance out any acute toxicity benefit << ‘cause the trial is negative for acute rectal toxicity).
3: and 4: True but discussed previously. One single event away from both being false.
5: p=0.087 from the results section as discussed above - not significant - and yet it made it to the abstract.
6: Per trial design, this conclusion is contradicted by the primary trial endpoint.
In closing, it is easy to read the paper and the abstract and MISS the fact that the primary safety / clinical endpoint was negative. That fact should have been front and center. If you want to caveat the results following a clear statement of a negative primary outcome, that is fine, but that did not happen.
Obviously, looking back, I’m unimpressed. I’m a bit surprised it made it through any real peer review in current form. I intended to cover all three articles in detail. But in my opinion, the others present less convincing data, so we’ll go more quickly through publications 2 and 3.
Final results of a phase III trial, publication 2 of the study patients, December 2016
This second study looks at CTCAE events and EPIC domains. This was not part of the primary trial design - new IRB - new duration extending to 3 yrs - participation by institution was voluntary. Not saying it is bad - just stating that I don’t believe it is what the title of the paper says it is - “final results of a phase III trial” - I see it as a secondary analysis with extended follow-up. (plus they opt to come back in a few months with yet another analysis so it isn’t “final results” on two levels).
CTCAE Events:
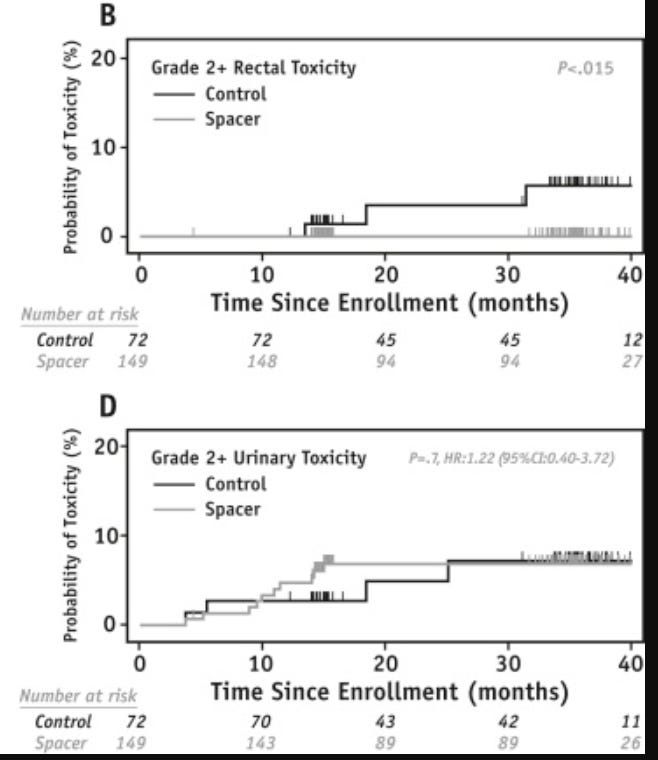
I included Gr2 and higher toxicity curves as shown below: There was a difference in Gr2 rectal toxicity 6% to 0% with a moderately strong p value of <0.015. This very well might be a valid, reproducible finding.
There was no difference in urinary toxicity for Gr 2 or higher. Gr1 toxicities are reported as different, but I personally find that data less convincing as Gr1 toxicities carry minimal clinical impact.
Initial QoL analysis:
I’ll summarize - sliced and diced 3 ways. GI toxicity was reduced with 2 of 3 p values showing significant improvements. GU toxicity was reduced with 1 of 3 meeting significance (and barely at 0.034).
According to the paper, after that analysis they noted the difference was larger at 3 years and so… they then assessed the “overall burden of QOL changes at 3yrs”. At this point, I’m going no deeper. You do not get 50 chances to analyze data to achieve p<0.05. But ultimately, they do derive p values of 0.0017 and 0.0049 so it must be correct. (read last sentence sarcastically as I rate these findings as a definite maybe).
I’m going to clip this paragraph from this publication. On the heels of the above statistics, I think it is important when we reach the 3rd trial.
The abstract of this article includes most, if not all, positive findings. 11 printed p values - 10 significant. Ok. We got p values, but not sure on much else. The strongest data appears to be the Gr2 rectal toxicity reduction with both CTCAE and potential QoL findings. But remember context, the original trial was negative for a clinical reduction in acute rectal toxicity. This secondary analysis argues maybe they missed something, but in light of the above noted issues, I even question this.
Paper 3: Sexual quality of life following rectal spacer.
The second “final look” as I call it. It is another look at the same 222 patients published this time in July of 2017. So about 7 months after publishing a secondary analysis that showed - their words “No significant differences” in sexual QOL, we have another analysis looking at sexual quality of life outcomes. Now we have a publication which states in the conclusion simply:
Conclusions: The use of a hydrogel spacer decreased dose to the penile bulb, which was associated with improved erectile function compared with the control group based on patient-reported sexual QOL.
Wait?? Seriously??
So what changed? Well, they trimmed the patient population opting to retrospectively apply this third “analysis” to the 41% with EPIC>60. In this subset, using specific (but not predefined) time points, they find marginal p values that I’d say, more often, just show trends towards better.
Conflicts of Interest Disclosures and Study Funding.
All studies supported by research funding from Augmenix, Inc.
Paper 1: 2 authors owned stock in Augmenix per article disclosures and a 3rd was a paid speaker by the company.
Paper 2: 5 authors disclose conflicts of interest with Augmenix.
Paper 3: 8 authors disclose conflicts of interest with Augmenix.
(In general, I have no issues with sponsored trials or being paid for work. Here, based on what I outlined, I thought the public disclosures are pertinent to reference.)
Final Thoughts:
So as a technology guy, I’m disappointed. Most protons programs have used a rectal balloon. Our facility transitioned from balloons to rectal spacing in 2016 (a few years before my arrival) and in moving here, I focused on the proton data and trusted far too much the “prospective randomized controlled” trial nature, the authors and participating facilities, and the resulting fancy marketing graphics.
Do I think rectal spacing has no benefit - likely no. Based on my personal experience in OKC, I think it probably helps a little bit to reduce rectal doses, and either rectal spacing, or protons, or really high prostate patient volumes help to limit rectal toxicity. But I also think it is important to constantly reassess data and honestly, the lack of impact in the MIRAGE trial paired with my current review of the original publication, gives me pause.
Based on my review of the data, I’d really like to see the multivariate analysis for rectal spacing regarding acute GI toxicity from the MIRAGE trial. And in the future, 3 yr GI toxicity data. If it doesn’t make a difference in SBRT prospectively, we should reassess.
And I now assume GU and Sexual toxicity is likely no different.
Trying to call balls and strikes - here is a quote from me from about 3 years ago - pre-covid - new at the center - and today after a trip down the rabbit hole, it was too positive.
I think the data for SpaceOAR is great and the procedure is pretty simple and straightforward with low risks. I think it is even a stronger compliment to proton therapy than with traditional radiation and that is why we using it here at the Oklahoma Proton Center.
Now with greater hands on experience, fresh eyes on data, and the MIRAGE data:
I think the original data for SpaceOAR appeared reasonable but on closer review and with the MIRAGE prospective data showing no benefit, I see it as optional even in the setting of SBRT. Its use still makes the most sense in higher dose per fraction settings. It potentially helps minimizing rectal toxicity following treatment by decreasing dose to the rectum, but it does carry risks that are not inconsequential relative to the potential for benefit.
Note: This data review is based on a 2023 review of past publications. I didn’t participate in the pivot trial. I haven’t talked to the authors. I obviously wasn’t in the “weeds” years ago. As always, if you have more direct context and disagree, please comment or contact me.
Post publication of this article: Twitter discussions generally agree with the declining value of SpaceOAR in recent years with advances in treatment technology. The other thought is that as we continue to decrease the number of fractions and push doses another 20% higher, rectal toxicity will play a more important role again. But at least to dosing of 40Gy / 5 fx SBRT with option for SIB to 42.5Gy, the data is for rather limited benefit (and I bet that is 99% of current treated cases).
REFERENCES:
Cost-Effectiveness of rectal spacing:
https://pubmed.ncbi.nlm.nih.gov/30342180/2019 Augmenix Pricing / Coding Information:
https://www.spaceoar.com/assets/SpaceOAR-Hydrogel-2019-Billing-and-Coding-Guide-Rev-J.pdfhttps://news.bostonscientific.com/2018-09-06-Boston-Scientific-Announces-Agreement-To-Acquire-Augmenix-Inc
RO-APM Physician Payment Model:
https://ascopubs.org/doi/full/10.1200/OP.21.00298MRI MIRAGE Trial Publication:
https://jamanetwork.com/journals/jamaoncology/fullarticle/2800541Systemic Review and Meta-analysis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7301230/Hydrogel injection reduces rectal toxicity after radiotherapy
https://pubmed.ncbi.nlm.nih.gov/27632342/Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015 Aug 1;92(5):971-7.
Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017 Apr 1;97(5):976-85.
Hamstra DA, Mariados N, Sylvester J, et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: Secondary analysis of a phase 3 trial. Pract Radiat Oncol. 2018 Jan-Feb;8(1):e7-e15.