We’re publishing one day early as ASTRO begins tomorrow.
If one were to casually peruse our literature, it would be a pretty quick summary for prostate cancer: SBRT is the path forward. Clearly where we all need to head. It is safe. It cures just about everyone. You know, 95% biochemical disease free survival across low, intermediate and high risk patients.
Ninety-two percent, 78%, and 38% of studies included low, intermediate, and high-risk patients. Overall, 5- and 7-year bRFS rates were 95.3% (95% confidence interval [CI], 91.3%-97.5%) and 93.7% (95% CI, 91.4%-95.5%), respectively.
But I think a recent conversation shows just how nuanced this path is today.
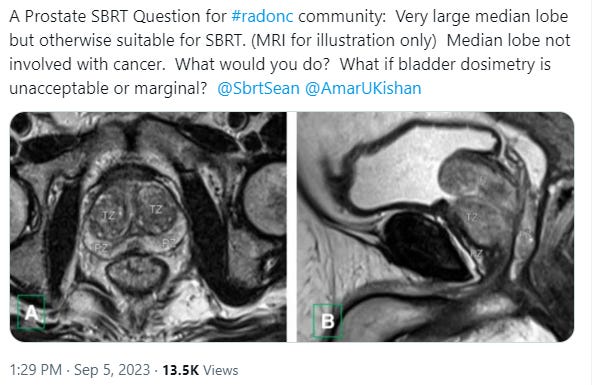
In simple terms, it goes on to discuss a case like this example pic and how to attempt to minimize bladder toxicity - and whether SBRT is even really recommended here - in a patient with more difficult anatomy? A good discussion with some good references brought in - recommended if you want a deep dive.
And remember, move to the headlines and you get great control. Go to NCCN, and there are no caveats - just standard of care for unfavorable, high, and even very high risk disease despite really limited data from the hands of few. Even the experts agree the data is limited and the approaches deserve caution. Yet the headlines storm forward.
Today, I firmly believe that there is far more nuance in the treatment of men with prostate than the open and common headline narrative. This lack of discussion of details is not helpful to our field. We need to understand what lies beneath the surface, for the safety and well-being of our patients. Again, for context, I like SBRT as an option. There is some great data out of high volume centers demonstrating excellence in outcomes. But from here, our goal seems to be to roll this out to much less experienced centers and that, to me, means we must have discussion regarding details of the data. So today, we dig.
It reminds me greatly of the days of my training - where we were beginning to push doses up from 66-70Gy to higher doses. At that time, there was tremendous nuance in the approaches that allowed for relatively safe dose escalation to more modern doses. Today there is far less nuance required. Honestly today, one can circle the prostate, add a standard margin, hit calc, and look for green checkboxes in the IMRT world. So long as your contours are decent, you should be fine.
In contrast, did you know that in the original MDACC dose escalation trial that, for the boost, the posterior block edge was 0.75 - 1 cm from the prostate - with no IGRT. No conebeam. No fiducials. No PTV. No extra margin for penumbra. If you include a PTV of 3-4 mm, dose was short posteriorly on target. We covered a non-moving CTV on paper without any real posterior margin. As I have said: Nuance.
Memorial was running the other dose escalation trial at the time and I am pretty certain that at some dose point they did something similar - the rectum was completely excluded from the field above certain thresholds. Nuance.
So this week is ASTRO in San Diego and many of us will make the journey out to the west coast and hopefully learn something. One of the items that I’m looking forward to is to see more data out of PACE B. It is the plenary session Monday Oct 2nd 2:10 - 2:20. Apparently five year results!
And just last week, I learned something new regarding this trial. And again, I’ve read quite a bit of our literature - obviously I’m not perfect and didn’t enroll on the trial but wow - I didn’t understand the fundamental dosing of PACE B. And I keep the 72 page UK document on my desktop for reference - literally.
How did I get arrive at my current thinking?
Well, I jumped into the SBRT prostate pool with both feet a few years back after reading the literature (ok… well maybe more headlines than I appreciated at the time) and truly believing that SBRT with protons might very well be a wonderful option - bringing in the benefits of several radiation oncology advances. I had been on the ground at a proton center for nearly a year and I had an SBRT proton protocol to follow with clear details (was enrolling men at the time on that RCT).
And then my first 15-20 patients seemed to clearly have more toxicity than I anticipated and their PSA kinetics seemed clearly different (ie did not go as low as quickly). So I slowed my transition and started my own database as I described in much more detail here:
Now about 4 1/2 years into my experience at a proton facility, I’ve spent the last year or so digging - trying to figure out if my gut was right or wrong on that small cohort and where I really think the data is today. This has been one of the focuses of this Substack.
And even within that story, I missed the dose recommendations beneath the headline. Maybe that speaks to an issue with me (haha), but I’m sure I read more on this topic than 95% of radiation oncologists and not just headlines but plenty of references from those papers. Perhaps some have far greater context if they have been in a dedicated GU program, but I’m gaining quickly at a proton center. So my guess is, I’m not alone in missing a subtle yet critically important dose detail.
And, just for those new to the site, I like dose, I like SBRT, I like SIB, brachy is a great tool and yes I like technology, with MRI and protons. There isn’t one path to great results - I think we have several options. But I do think, of the options above, the most nuance lies within the shortest fractionation approaches.
Wordsmith: Nuance or Narrative?
I titled this article with the word nuance, but one could choose the word narrative. Even the top line claims - 95% control I believe will be proven wrong for intermediate risk disease and higher. I don’t believe many people think that PACE-B will hit 95% disease control - even though it ONLY treats low risk and more favorable intermediate risk disease.
(and if it does look immediately at follow-up duration - it should be nearly 84 months (I’d say at least 75 months) median to report 5 year Phoenix data. This isn’t a “Mark Storey SubStack rule”, this is original definition rule from the original creators to minimize time bias in an outcome measure so clearly linked to time. Go read the original Phoenix definition document)
If you read the thread on X with the large median lobe case, you will see that we have nuance in technique that relates to our attempts to limit toxicity. At the same moment, we have a narrative in our publications and advertising as we push the field towards less fractions almost to a point of disregarding data. I’ll explain.
What does the data show to this point?
Some degree of higher acute toxicity. How important this difference is I think is a pertinent question. Paper: (PACE-B): 2-year toxicity results
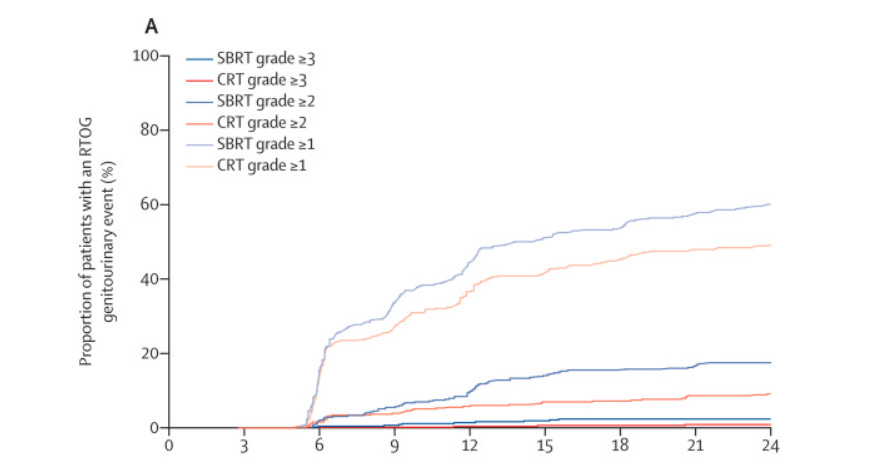
Cumulative grade 2 or worse genitourinary toxicity rates were higher with SBRT than with CRT, as assessed by both RTOG and CTCAE criteria. At 2 years, cumulative incidence rates of RTOG grade 2 or worse genitourinary toxicity were 10·6% (95% CI 8·0–14·0) for CRT (45 events) and 18·3% (14·9–22·4; 75 events) for SBRT (HR 1·80 [95% CI 1·25–2·61]; log-rank p=0·0015; figure 3A). Corresponding figures for CTCAE grade 2 or worse genitourinary cumulative toxicity were 19·8% (95% CI 16·3–23·9; 84 events) for CRT and 32·3% (28·0–37·0; 132 events) for SBRT (HR 1·73 [95% CI 1·32–2·28]; log-rank p=0·0001; appendix p 13).
Hazard rates of 1.8 and 1.73 for SBRT with respect to cumulative Gr2 toxicity. In places, this type of toxicity difference might remove the new treatment from consideration. Or at least the enthusiasm for the approach, but not here where the goal is to clearly push in this direction.
And further: this is really a consistent finding of all prostate cancer hypofractionation to SBRT approaches. They all demonstrate higher toxicity as you shorten the course of treatment yet have been “proven” equivalent within non-inferiority trial settings. At what point is the toxicity “relevant” vs. the efficiency gains and less number of trips is the more difficult question, but in simple terms acute toxicity increases. And here in this randomized data, we see that simple point illustrated once again.
Early toxicity translates into higher late toxicity.
This association also remained significant in multivariable analysis. Grade 2+ baseline GU symptoms were also associated with grade 2+ late urinary toxicity in both univariable and multivariable analysis. Overall, acute toxicity is an important predictor variable for late GU/GI toxicity after localised prostate radiotherapy using SBRT and CRT.
And I think this is good information to consider. While most of the toxicity differences abate with time, there is a link in this randomized dataset of acute toxicity leading to an increased risk of late toxicity. This is randomized prospective data linking acute toxicity to late toxicity.
PACE is looking to actually reduce dose to the periphery of the gland in future trials - obviously they see real toxicity.
Per a TAP podcast (I think specifically this one), PACE is now looking to lower whole gland dose to 30/5 fractions - I assume this is directly related to some level of toxicity difference. If you see no toxicity, you wouldn’t tighten up the margins / reduce the peripheral dose.
From a technical standpoint in that setting this will be done by removing the margin - ie eliminate the PTV, but less dose to the periphery is the end result of either method you choose to use.
PACE-B: More Things to remember:
It a relatively favorable cohort of intermediate risk disease
I’ve reviewed this before but PACE-B is low risk disease and a pretty favorable group of intermediate risk disease. Really there is no unfavorable disease within the results so low risk outcomes should be north of 95% and the drop from there is all due to a cohort of more favorable (not exactly NCCN) intermediate disease.
As a refresher, here are MDACC results - in FIR subtract at least 2%-3% due to a rather large usage of ADT. Follow-up is a full 7 years.
Watch the follow-up
Remember, for Phoenix definition, we should be looking 2 years prior to median follow-up. So if median follow-up is 5 years, then one should focus around the 3 yr mark for the KM curves. The 5 year results will slide - often about 5% with longer duration follow-up. (I do think if results are well north of 90%, they will slide less - say 3% or so, but time always brings down the results).
It is a non-inferiority design
To quote the design document: PACE-B: 858 patients provides 80% power to rule out a detriment of at most 6% (non-inferiority margin) in biochemical or clinical failure at 5 years assuming the proportion of patients biochemical or clinical failure-free is 85% in the control arm (critical hazard ratio = 1.45). So if the standard arm is 87% control, non-inferiority would be anything better than ~81%. (That said, I predict the SBRT will be several percent higher for control).
It should be able to more definitively put to rest any linac, Cyberknife discussion
Most intermediate risk disease in our literature is from Cyberknife - not bias, just reality of the data we quote. This trial has good populations from each program and should put this bit of trivia to rest.
In fact, the original design of PACE-B limited the SBRT arm to Cyberknife only. This was changed to later allow linac based programs to participate. It includes some MRI linac patients but I believe less than 15.
Margins are different in the arms - significantly different
5-9mm in the hypofractionated arm (3-7mm posteriorly) vs 4-5mm in the SBRT arm (3-5 posteriorly).
Remember in the Mirage trial a 2mm difference in margins drastically reduced bladder toxicity.
So 2mm created a large reduction in GU toxicity. In contrast, in the PACE-B toxicity results we are seeing SBRT (despite a 2.5mm margin advantage) result in higher toxicity. If MIRAGE is correct, acute toxicity due to margin differences in PACE-B should be much larger in favor of SBRT. In contrast, what we see, is that toxicity is significantly higher despite an even larger difference in margins. And therefore the reality becomes: if tighter margins are ok, then the GU toxicity differences are, in reality, far more different.
Yes the difference dissipates with time but there is a real difference in cumulative toxicity in favor of hypofractionation despite a real disadvantage in margins. And again, this translates to late toxicity.
Headline dose and delivered dose are VERY different
Do not just roll out uniform dosing SBRT for prostate cancer the week after the meeting thinking you are mirroring a PACE B approach without due diligence.
This trial is generally reported as 3625 cGy vs. 7800 / 39 or 6200 / 20. But that oversimplifies the SBRT arm. While the standard arm is what one would presume, on the SBRT side, there is a PTV dose of 3625 to 95% of PTV but a CTV dose of 4000 cGy to 98% of CTV. I understand that the CTV dose is approximately 40Gy in the SBRT arm (personal discussions), but this has not been publicly reported.
In the data, look for the number of patients achieving both dosimetry goals in the SBRT arm. It is important - I’ll assume it is very high number so I’ll reserve additional commentary should it not be.
But this also drives and actually enlarges the effective margin differences. I’ll explain.
The standard arm prescribes dose to the PTV without purposefully heating up the CTV relative to it - the puts the margin of the PTV on the top part of the shoulder of the dose distribution. In contrast, the SBRT arm lies on the steep “SBRT” part of the dose distribution - already at 90% or below.
So if you have any concern about EPE or tight margins, the dosing schedule further amplifies that effect. At least another 1mm difference between the two arms. Meaning for the bladder - we are closer to 4.5mm difference in margin in favor of SBRT yet it is still losing - in a measurable way - to the acute toxicity of the standard arm.
Summary:
I like SBRT. I think high volume programs have proven great biochemical results using short / fast approaches. I actually think, with a prostate dose of 40Gy, it will approach being proven superior with respect to control.
But it appears to have significant increases in toxicity - whether those negate the benefits for you and for your patients is a difficult discussion. After all, even though the cumulative toxicity numbers are different, the absolute difference is small and SBRT is, in general, well tolerated.
At 24 months, RTOG grade 2 or worse genitourinary toxicity was seen in eight (2%) of 381 participants assigned to CRT and 13 (3%) of 384 participants assigned to SBRT (absolute difference 1·3% [95% CI –1·3 to 4·0]; p=0·39); RTOG grade 2 or worse gastrointestinal toxicity was seen in 11 (3%) of 382 participants in the CRT group versus six (2%) of 384 participants in the SBRT group (absolute difference –1·3% [95% CI –3·9 to 1·1]; p=0·32).
I like where we ended today - I think close looks follow-up duration as well as at the margins and dosing for SBRT arm in PACE-B are important to remember before a quick 10 min presentation on 5 year cancer outcomes.
Looking forward to the data. Kudos to all involved in such a great project as we push for better.
Early results leaked / announced well prior to the session:
I’ll come back with commentary later and assess expectation vs. results.
REFERENCES:
Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial
https://pubmed.ncbi.nlm.nih.gov/31540791/
Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial
https://pubmed.ncbi.nlm.nih.gov/36113498/






OC-0509 10-year outcome of ultrahypofractionated stereotactic RT from two multicenter prostate cancer trials
R. Meier1, I. Kaplan2, D. Bloch3, R. Chen4, B. Kane5, G. Henning6, S. Woodhouse7, T. Royce8, C. Cotrutz9, D. Fuller10
1Swedish Cancer Institute, Radiation Oncology, Seattle, USA; 2Beth Israel Deaconess Medical Center, Radiation Oncology, Boston, USA; 3Stanford University, Biomedical Data Science, Stanford, USA; 4University of Kansas, Radiation Oncology, Lawrence, USA; 5Community Medical, Radiation Oncology, Fresno, USA; 6St. Joseph Mercy, Radiation Oncology, Ann Arbor, USA; 7Carle Cancer Institute, Radiation Oncology, Normal, USA; 8UNC Health, Radiation Oncology, Chapel Hill, USA; 9Swedish Cancer Institute, Swedish Radiosurgery Center, Seattle, USA; 10Genesis Healthcare Partners, Radiation Oncology, San Diego, USA
Purpose or Objective
We previously published 5-year outcomes in two large multicenter trials of ultrahypofractionated stereotactic radiotherapy for organ-confined prostate cancer. Randomized trials have subsequently demonstrated that, with similar follow up, ultrahypofractionated radiotherapy is a suitable alternative to conventional fractionation. Since data beyond five years are lacking, we now present combined 10-year survival and late toxicity outcomes from these two prospective trials.
Materials and Methods
Downloaded for Anonymous User (n/a) at Providence Health and Services Oregon and Southwest Washington from ClinicalKey.com by Elsevier on October 12, 2021. For personal use only. No other uses without permission. Copyright ©2021. Elsevier Inc. All rights reserved.
S392
ESTRO 2021
39 centers enrolled 569 patients with prostate adenocarcinoma: 284 with low-risk and 285 with intermediate- risk disease. 101 patients had unfavorable intermediate-risk tumors (Gleason 4+3, two unfavorable risk factors, and/or ≥50% biopsy cores positive). All were treated with a non-coplanar robotic stereotactic platform using real-time tracking of implanted fiducials. Two dose regimens were used: 40Gy in 5 fractions of 8Gy, and 38Gy in 4 fractions of 9.5Gy. Adjuvant androgen deprivation therapy (ADT) was not allowed. Early (within 3 months of treatment) toxicity and 5-year survival outcomes have been previously described; we report outcomes in patients consenting to study participation beyond 5 years. Toxicities were assessed using Common Terminology Criteria for Adverse Events (CTCAE) version 3. All reported rates are actuarial, using Kaplan-Meier method. For relapse free survival (RFS), failure included initiating salvage or systemic therapy, local/regional/distant failure, or biochemical relapse using the “nadir + 2” definition.
Results
Median follow up was 8.1 years, with 109 patients followed for 10 years. There were no grade 4-5 toxicities. Actuarial 10-year grade 2 and grade 3 late GU toxicity rates were 14.9% and 2.2%, respectively. The 10-year late grade 2 GI toxicity rate was 3.6%; there were no grade 3 GI toxicities. For the entire group, 10-year overall survival rate was 88.1%, local failure rate was 2.5%, and RFS rate was 92.0%. 10-year RFS for low- and intermediate-risk groups were 98.5% and 85.8%, respectively. 10-year RFS for favorable and unfavorable intermediate-risk patients were 91.5% and 75.3%. No statistically significant differences in rates of toxicity, survival, local failure, nor RFS were observed between the two dose regimens.
Conclusion
10 years following treatment of organ-confined prostate cancer with ultrahypofractionated robotic stereotactic radiotherapy, toxicity rates continue to be minimal, with few additional events observed beyond 5 years. Overall survival, local control, and RFS rates remain favorable at 10 years, confirming stereotactic radiotherapy as a suitable option for low- and intermediate-risk prostate cancer.
Check out figures 17-20 Table 18c-d Table 23 Fig 22 etc and most importantly Fig 21c
Cyberknife does Better than conventional linacs
Supplements The Lancet Oncology article 2022