Head and Neck Cancer: Overall Survival Benefit with Protons...?
Updates from PTCOG in Argentina. An initial look at the randomized prospective data.
An Overall Survival Benefit in Head and Neck Cancer?
Steven Frank MD presented overall survival data from the US multi-institutional randomized prospective head and neck study comparing proton therapy to IMRT demonstrating a statistically significant improvement in overall survival:
Proton Therapy increases Overall Survival in the treatment of Oropharyngeal Cancer of the Head and Neck.
This is now a data driven level 1 evidence statement.
To put this into perspective, I believe that ONLY brachytherapy for cervical cancer and SBRT (several sites) have any data suggesting an overall survival benefit when compared to existing / alternate approaches. Not IMRT, not any device, not IGRT, not tracking or adaptive. Essentially near nothing. Heck it is often tough to show that radiation vs. none impacts survival in many sites.
So within that context, one needs really ask - really?
Did protons really demonstrate a true and reproducible survival advantage?
And here, I’m less certain.
To date, we have pretty limited information. The full publication is pending and that likely adds significant clarity. That said, my guess is the paper doesn’t sway many people too far from where they land today. Realistically, I personally believe we’ll need the validation of the data in the large randomized trials that are coming from Europe and the pre-planned patient data driven meta-analysis from this and DAHANCA and TORPEdO for many to land on this altering standard of care.
I wasn’t in Argentina - so this is simply my read of some of slides - quick highlights - no direct conversations regarding the data at this time.
***Please consider a like or share or comment. Helps to support the free site. Thanks***
If you subscribe, Substack now automates a pretty good podcast track that becomes available via your subscription in the app for my entire site - AI but a nice option.
IMRT: Intensity Modulated Radiation Therapy = Photon based treatment.
IMPT: Intensity Modulated Proton Therapy = Pencil Beam Proton treatment.
Below you can see a breakdown of the trial. Simply stated, there is massive crossover. 66 in the IMRT arm treated with IMPT. 35 in the IMPT arm treated with IMRT.
So the trial ends up with three different analysis that need to be considered.
Intent to treat (the 219 IMRT vs. the 221 IMPT).
As-Treated - numbers not shown. Likely swap of the 66 and 35 respectively (resulting in 171 IMRT vs. 226 IMPT)
Per Protocol analysis with far fewer patients. (136 IMRT vs. 160 IMPT)
In the Abstract Only release from May of last year, somewhat amazingly, all three of these analysis trended very much in the same direction with a similar magnitude of benefit for all primary and secondary outcomes despite rather large differences in the numbers of patients in each analysis. This presentation increased follow-up duration and I believe largely focused on overall survival data. The other metrics like progression free survival, feeding tube and weight loss data seem to be better presented in the abstract.
Here, I assume the prior trends remain largely intact but these types of questions will require the full publication.
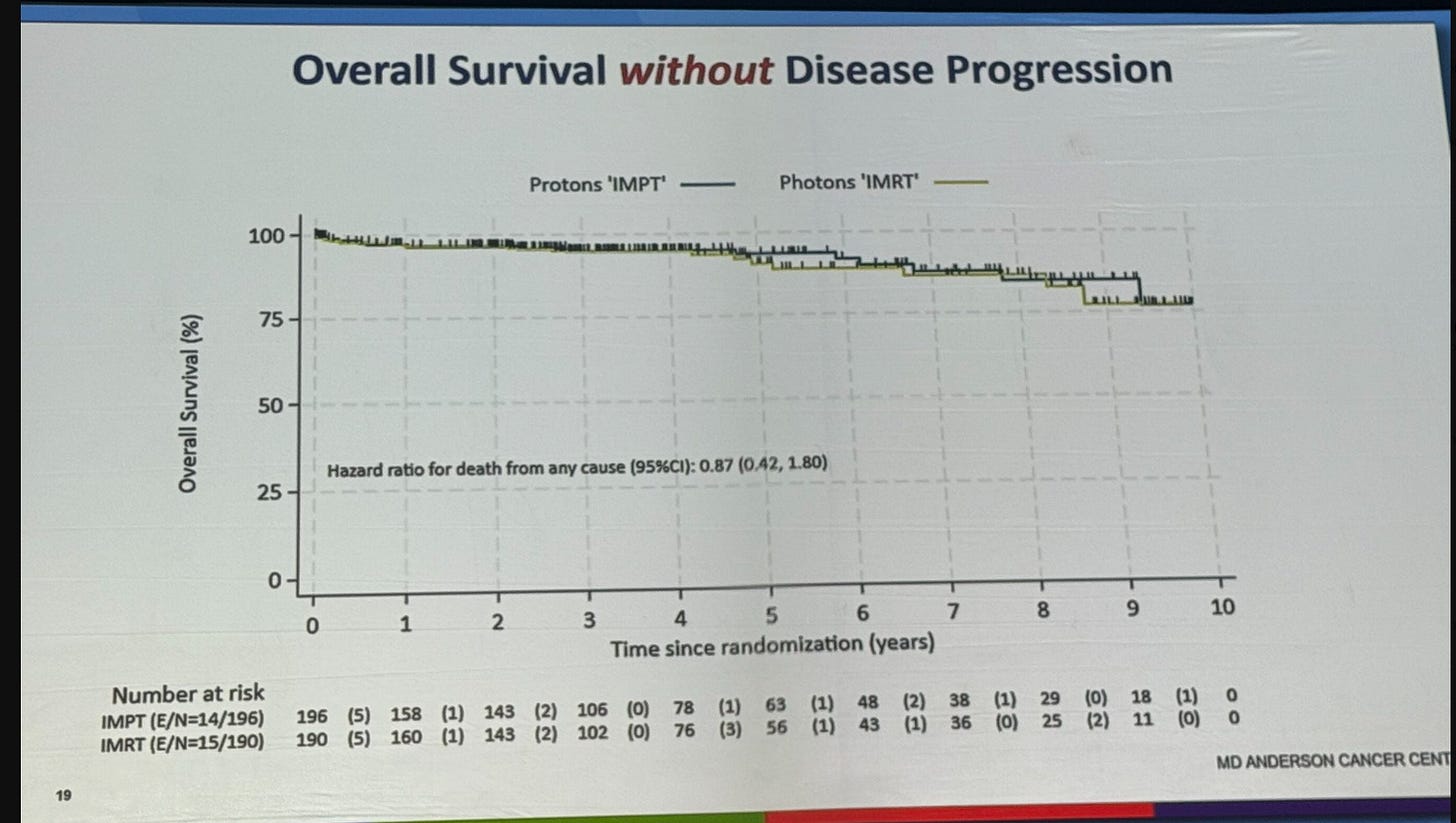
Beyond crossover, the second appropriate critique is the following - the curves seem to carry most of the separation years down the road - i.e. after 3 years (see above). And then these two slides below were presented showing the improvement lies within the small cohort with disease progression.

Again, I wasn’t there. Don’t know what was said, but this certainly isn’t what I would have guessed. At least to me, this seems unexpected.
Now, there is some relative difference early - the three year OS hazard rate seems to be about 0.78. But here it increases to 0.47 at five years. Personally, I’d like to see another 40 people cross the 5 year mark (at least half of 80% survival per arm) to really trust that metric but this was a large multi-institutional trial and almost certainly these analyses were pre-determined. But for me, I think there are too many early censors for me to really trust / lean into an overall survival improvement on a debate stage.
And the salvage impact only really “makes sense” if somehow a later stronger baseline contributes significantly to salvage treatment. But it also brings far more opportunity for confounding in the salvage approaches.
Below are Dr. Franks Summary Conclusions: Read twice as they are quite strong.

In follow-up questions online, lymphopenia (a known predictor of overall survival) significantly favored proton therapy (a common finding in our modern literature) and was felt to a contributor to the higher salvage rate and better survival for those who recurred. Again, I think this is why we need the paper.
Brief Note on “Non-Inferiority”:
Almost certainly you’ll see people point to the “non-inferiority” aspect of the trial and try to dismiss the relevance of protons outperforming IMRT. If interested, read my full article on this point back from over 2 years ago. Briefly, the clinical investigators wanted a superiority design - tried multiple approaches, but an NRG review mandated a non-inferiority design.
Non-inferiority Trial Design (REFERENCE SERIES)
And so when I look forward to the US OPC data, I anticipate that these issues and types of conversations will suddenly surface rather broadly throughout our field. I anticipate large blocks of physicians will argue quite strongly against the trial, in part due directly to the non-inferiority trial design. And now, as a reader here, you might understand that discussion a little better.
My Summary: As of Abstract and Presentation
Protons appear superior regardless of whether the overall survival data is replicated.
This trial was designed to evaluate Progression Free survival as primary endpoint in a non-inferiority fashion. From the Abstract, both the Per Protocol and As Treated leaned towards better control with protons. (HR=0.85 p=0.009 and HR=0.88 p=0.007). I assume these trends remain relatively intact. (I assume that p value represents a non-inferiority test - not a superiority of control with protons test).
Pre-specified secondary metrics were:
Overall survival - favors protons - whether p is significant or not - anyone would “choose” the top curve.
Malnutrition - significantly favors protons.
Feeding-tube requirement - significantly favors protons.
So even if you don’t think the overall survival benefit is reproducible, the trial appears to have leaned towards protons in the primary metric and every secondary outcome. Statistically, it is correct to say not inferior with respect to the primary metric, but all four, at least, lean towards protons.
And unlike in chemotherapy or drug intensification trials, there is no risk here - at least presented to date. Zero. Toxicity is reduced. Cost-analysis are presented as improved (they’ve published / presented this several times previously out of the MD Anderson system and it is reiterated here with all-caps). The “downside” is a referral. The upside is overall survival.
It will be interesting to watch the impact. We accept so many trials regarding systemic intensification on progression free improvement or event free improvements with border line overall survival benefits - where the benefits come with 30% Grade 3 or higher toxicity. Here is the opposite - improved outcomes with less toxicity.
Do I fully believe the benefit in overall survival? No and I probably won’t even after the full publication. Do I believe it trends in that direction? Maybe. Which outcomes appear superior? Protons - not equivalent - superior. Do I think this validates my thinking in moving 7 years ago for proton therapy specifically to try and improve the outcome in my head and neck population of patients? Absolutely. Today, would I personally travel out of state for treatment based on this data? Yes.
As I’ve said for the past few years, the European trials will be important to help validate the magnitude and scope of improvements. I expect them to be far cleaner trials with respect to crossover and therefore better limit confounding. The three trial meta-analysis (covering this trial, TORPEdO, and DAHANCA) will serve as the gold-standard moving forward in my assessment.
Finally, just to be clear: if in this trial landed in the opposite favoring IMRT for progression free and OS maybe even with some subtle improvements in toxicity on the proton side, the entire arena of protons within radiation oncology would have needed to sit down and pause. And hopefully I would have written something stronger than today’s article in the opposing direction.
That said, I *think* what you are seeing in recent years is that the shift to pencil beam is creating real benefit in high risk patients even compared to great modern IMRT - from esophageal, to lung, to now head and neck cancer. These aren’t the proton plans of 2013.
RADCOMP ACCURAL COMPLETE!!
Second big news is: RadComp has completed accrual of 1238 patients. This is a large volume (>1200 patients) randomized multi-center breast cancer trial comparing protons to photons. Below are highlights.

Note planned dates: Health Related QOL in August of 2025, but then the long-term MACE outcomes are not available until 2032.
My clinical guess is that protons are likely are WORSE if anything in the August of 2025 report - protons simply have more skin reaction if you aren’t really careful / aggressive with planning (even then still more IMO). That report will start an onslaught of negative reports. We’ll then need to wait about 7 years for the cardiac data to surface. I really have no doubts the cardiac data will favor protons - my opinion but again, over 6 years into this with 25 years of cardiac dosimetry history.
This trial will be an exercise in patience. I expect this to be a long and painful journey as many will exuberantly claim - far too early - the demise of protons for breast cancer. And ultimately, if the cardiac data fails to show improvement or the local regional control data shows increased failures, then protons will have failed. But watch - many will make that call later this year, when the long-term reality of the study might very well be the exact opposite of early QoL data.
In Summary:
Crazy data in the head and neck space with IMPT likely landing a big win (pending full publication). Whether or not most view the overall survival benefit as “real” will be a point of reasonable debate. My guess is most will wait for the European trials and the meta-analysis. But don’t kid yourself, this single trial starts to make out-of-state referrals / requests for referrals for many head and neck cases very appropriate. And, if the overall survival difference is validated out of Europe - what’s that quote?
“Houston, we have a problem.”* - a big logistical US healthcare access problem.
*Note: a common misquote of the actual statement, but it fits better.
As always, author of one. If you see an issue or error, please comment.
Addendum 6.26.2025: Two slight tweaks in wording - very subtle. I don’t think the trial was formally an NRG trial. I *think* MD Anderson eventually ran the trial but the structure was set via a cooperative effort with NRG during trial development.









This is a curious finding, as the more numerous 3-D versus IMRT trials never did consistently show a survival benefit to IMRT over 3-D for H&N cancer, despite significant toxicity reduction for IMRT. I would argue that 3-D to IMRT is a bigger leap than IMRT to protons.
1. serious conflict of interest (pro? ton$ !), and $eriou$ly no joke
2. impossible to logically explain that regardless of subset analysis, "proton$ wins".. please
3. survival benefit? maybe* toxicity improvement leading to survival benefit? maybe? nah
4. I'm not buying it, and its unrealistic to expect average H&N treatment to suddenly result in the popup of ton$ of expen$ive proton centers for a cancer making up 3% of the cancer pop.