Prostate Cancer: Add Chemo? No thank you.
An ICECaP study recommends chemo for high risk low PSA cancers. I'm far from convinced.
Within my first 5-10 minutes looking at the article, a lyric popped into my head.
“Don't believe everything that you breathe” - Beck
Today we’ll look at a paper out of a large collaborative database with big name authors. I would think I should have read it and thought - “absolutely” or “that’s interesting!” But I didn’t. And ultimately I disagree pretty strongly with the choice of wording in the final conclusions and believe it actually puts some patients at risk for over-treatment. Interesting concept perhaps, but it overstates the data - favoring chemo and undervaluing radiation - that is my assessment.
As always, author of one. I’ll present my view and you should read and assess where you land. Always easier to write simple supportive reviews, but evaluating literature is critical to what we all do each and every day.
Most of the time I try to focus on good, positive stuff. But with today’s paper, the implication is HUGE for those affected - Adding up front Docetaxel to high risk, low PSA prostate cancer - and it got some pretty big coverage. Here is an example post that caught my eye.
It had a link to the article with big names and nice curve showing, what appears to be, a pretty dramatic improvement. It caught my eye and I jumped into the rabbit hole to look around.
And ultimately, the post isn’t “wrong” - it says “might benefit” but wow… today, we’ll look at the underlying paper and you can decide if you think you would consider this approach or not.
I want to start out by saying this is a large topic with a variety of studies that one can point to. My approach in these big topics is generally to walk through the article and review what THE AUTHORS reference in their words / discussion. That’s the approach we’ll take today. If anything, that should bias me in favor of the results.
The author of the post above came back and affirmed that he is now at least considering inclusion of treatment intensification based on this data - he implied off trial, current patients. And so if this data is that valuable - maybe altering first line treatment - we should look at it.
Today’s Article: Mortality Risk for Docetaxel-Treated, High-Grade Prostate Cancer With Low PSA Levels A Meta-Analysis
The headlines cover a lot of great buzzwords of the day - primarily the “individual patient data meta-analysis”. And it utilizes a great database of randomized controlled studies named the: ICECaP collaboration bringing “big data” and “big authors” to the table.
And the “take home” message is this: an improvement in prostate cancer specific mortality. Well, maybe…
At 10 years, your all cause mortality risk is less than HALF if you were able to get the drug doxetaxel.
Which begs the question - why it is so different from the randomized data for this drug that is sometimes favorable, sometimes not so much?
And importantly, which should you trust?
The question asked is really quite reasonable:
Is there a subset of men who might benefit from intensification beyond primary treatment and ADT?
And its an interesting approach to that question - basically asking if some high grade tumors don’t produce as much PSA as they should and therefore if you potentially look at low PSAs, you might find some that benefit.
Ok, so we are focused on a “de-differentiated” population
But how big is this cohort? The quote below from Dr. McBride is NOT related to the paper, but it flashed into my mind as I looked at this data. The odds of seeing a really de-differentiated tumor in the primary setting is really low. Like really, really low. Like in my last 300-400 prostate cancer patients, I really can’t recall a SINGLE <4 PSA where the biopsy just showed large volume disease and the MRI confirmed high volume, high grade disease. As I think… maybe one…. maybe… So I fall in line with Sean here - it is really extremely rare.
This just isn’t something I think I see - at a proton center - working with about 15 urologists - I still don’t see it.
Let Me Check Myself: Off to my Database:
So rather than me just making up what I think, I’ll open the database. Of ~300 prostate cases, I have one high risk case with PSA<4. Ironically he had limited disease on biopsy and a low Decipher (6% risk of metastatic disease at 10 years) with a possible 7mm region on MRI. I honestly doubt he is really within the hypothetical cohort example case. And then clinically, he is doing well - a couple of years out - after refusing ADT, treated with radiation alone once again arguing quite strongly that I have zero de-differentiated cases that meet these criteria for inclusion in my database. And that patient, probably pretty happy today about avoiding ADT and systemic chemotherapy.
So yea, I just don’t see this scenario very often - “extremely rare” would be consistent with my assessment. Well less than 1% of my ADT recommended patient population.
Ok… Let’s focus and look at the data and what they did.
Hmmm.. ok. Another important pause is required. They highlight two randomized studies and while they didn’t have the data, the results of the trials are in fact available. So let’s look at those and see what they showed in a cleaner look at this question.
The study included 298 patients randomized following prostatectomy to either standard treatment or adding docetaxel.
Conclusions:
Adjuvant chemotherapy in high-risk prostate cancer using docetaxel and prednisone did not lead to statistically significant improvement in PFS for the intention-to-treat population as a whole. The analysis was challenged by lower power due to accrual limitation. Subgroup analyses suggest potential benefit for patients with Gleason grade ≤7 and stage ≥ pT3b (ClinicalTrials.gov number NCT00132301).
Note: it didn’t even show a real difference in progression free survival none the less, anything survival related. It did trend towards a PFS benefit but in a completely different cohort than in this study - actually quite opposite (low Gleason scores).
Ok, second trial.
This trial is much closer to relevant, in that, it is a radiation cohort. The trial looked at radiation and ADT plus or minus 6 cycles of docetaxel in 376 patients. It did include 4+3 disease and higher PSAs but it again shows no hint of benefit.
The 5-yr estimated biochemical progression rates were 31% for arm A (chemo arm) and 28% for arm B (avoided chemo). Febrile neutropenia occurred in 16% of the docetaxel patients. No deaths were related to the docetaxel treatment.
Conclusions:
Adjuvant docetaxel without prednisone did not improve BDFS after radical RT with ADT for intermediate- or high-risk PCa.
So again, we see no benefit - not even in progression free survival (3% worse). And nearly 1 in 6 patients had febrile neutropenia. Thankfully, no deaths related to the intensification.
My Pre-Analysis Summary of Broader Context:
No trial is perfect, but here we should need a pretty darn high bar in anything looking backwards trying to do a “meta-analysis.” In the past, just using what they quote, we have TWO negative randomized trials in the modern era showing really very little suggestion of benefit.
In fact, per the introduction, there are 7 trials and only 1 possibly points to a possible rationale - a “hypothesis-generating postrandomization” yielded a hazard rate of 0.33. And if you dig on that point you will understand this was a non-planned exploratory analysis with 27 total men in that cohort. And worse, the hazard rate magically jumps from 0.27 (less than PSA 4) to 1.51 for PSA 4-20 back down to 0.6 for the 90+ men with PSA >20.
Do you trust a magical dichotomous evaluation of a continuous variable that looks at a 27 patient cohort? I do not. Yes, there are some historical data that one can argue for the “de-differentiated” rationale, but consider this. This argues that, back in day, when we “chose” PSA of 4 or less - that in fact, we landed on a magical number that divides docetaxel benefit by a factor of 5.5x times for high grade disease - that a high grade patient with a PSA of 3.2 is 5.5x times as likely to benefit as a PSA of 6. I seriously doubt that.
But that argument, according to the authors, is a main component of their rationale for this analysis.
Moving along:
First, we have data from 2598 patients in the ICECaP database. They then find 145 patients in 4 of the trials that are eligible. They say 6.6% because they cut the 2598 to 2184 but whatever - say 5%-7%. That means I should have ~10 patients in my database - just looking at the 150 or so that should be ADT eligible. As we’ve discussed, I have 0 or maybe 1 vs. and expected of 10. That screams that this analysis doesn’t relate to what I see in my clinic today. I encourage you to look through your patients and see how many fit the simple criteria of PSA<4 and Gleason 8-10. If you practice in the US, I bet it is quite a bit less than 5%-7%. This is yet another warning flag I see.
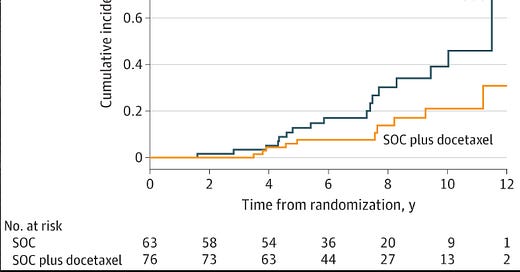
And then, from this cohort we get the survival curve graph that I showed at early in this post.
A reduced but nonsignificant risk of ACM (HR, 0.51 [95% CI, 0.24-1.09]) and PCSM (sHR, 0.42 [95% CI, 0.17-1.02]) was associated with patients randomized to SOC plus docetaxel compared with SOC.
Note: both have confidence intervals that cross 1. These are both non-significant findings when you adjust for covariates. They do have a significantly improved hazard rate reductions but typically not regarded as a significant finding.
Then they did an extra analysis limiting the analysis to only those with an ECOG Performance status of 0 (this removes 6 people) and arrived at a significant finding.
Then cut to the conclusions:
Conclusions and Relevance Adding docetaxel to SOC treatment for patients who are in otherwise good health with a PSA level of less than 4 ng/mL and a Gleason score of 8 to 10 was associated with a significant reduction in PCSM and therefore has the potential to improve prognosis.
Wait, Why did they look at ECOG 0?
Well, they say this:
Furthermore, in a prior study of men with intermediate or high-risk nonmetastatic prostate cancer,20 only men with no or minimal comorbidity appeared to benefit from the addition of ADT to radiotherapy with respect to OS; those with moderate to severe comorbidity did not.
Another reference to track down. After all that seems quite pertinent - it is the single largest study that represents the rationale for the look at ECOG 0 patients as a subset based on the authors of the paper. And that analysis flips this from not significant to significant.
Well, it is a trial from 2008 from one of the authors that used a far more precise mechanism for parsing comorbidity risk.
a comorbidity score was assigned by the principal investigator (A.V.D.) using the Adult Comorbidity Evaluation 27 (ACE-27), a 27-item validated comorbidity index for use in patients with cancer.
It divides patients into groups from 0 to 3 - and the paper then used 0 and 1 together and excluded 2 and 3. So just quickly, not ECOG and not two categories included, but a jump to just ECOG 0 in this analysis summarized quite broadly as “good health” in conclusions of the paper.
And by the way, in the current study, the hazard rate for ECOG 1 vs. ECOG 0 was 7.2 for prostate cancer specific mortality - simply a massive effect - one that I would bet, would not be reproduced in that very large database.
So just to summarize, rather than belabor the point too long on “good health”: the goalposts were different, the measuring sticks were different, and the parsing of the cohort was therefore different. The historical hypothesis generating “reference” removed half the patient population and the current analysis removed 4%, but hey, the p value is significant so the 4% must be correct and strong enough to HIGHLIGHT in the conclusion with a strong claim of improvement for “associated with a significant reduction in PCSM” with a vague poorly defined descriptor of “otherwise good health”. And remember, this whole thing works because those 6 patients did really poorly.
Summary:
When you have important, large datasets and you have strong, well known authorship, in my view, you really need to be careful with the work and wording that you produce. To me, this paper fails to meet that standard. It overstates the benefit and a few different choices would likely destroy any “statistical significance” in the findings.
Ironically, I find the question interesting. I think it might be true, but if you stop and dig in the weeds of this one, I’m honestly surprised it was accepted as written. The discussion is actually quite appropriate, just the conclusions are far too strong. And unfortunately, most will only read the headlines and abstract - tis reality.
At nearly every place I opted to look deeper, the work lost strength in the merit of the analysis. And ultimately it leads to the tweet at the top and I feel quite confident that some men will receive docetaxel for disease where I think the data is no where close to strong enough for that approach off trial.
Re-write the conclusion to below and I’m far less frustrated.
Conclusions and Relevance There is a potential signal within the data showing that adding docetaxel to SOC for the patients in otherwise good health with low PSAs and a high Gleason scores might offer benefit. Further study is indicated to determine whether this possible improvement in PCSM can be reproduced.
If you write it more in line with my re-write and someone picks it up and runs with it on a social media site, that is their problem, but when the original authors leave it open to extent that I see in this paper, it falls back upon authors and honestly, the journal and reviewers. I can almost guarantee you that if this was a no-named author submission with an unknown database, it would have been toned down or just flat-out rejected.
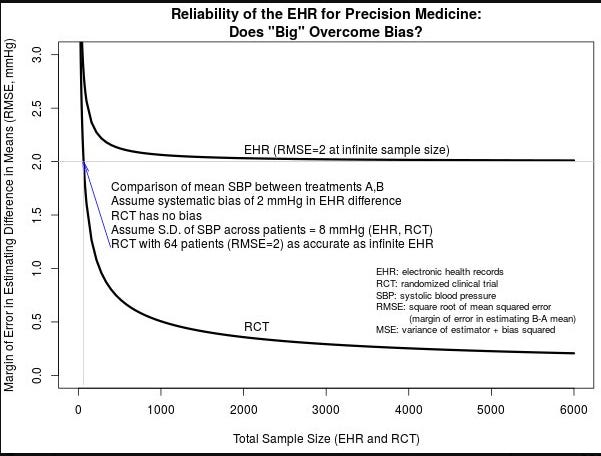
I’ll finish with this stats graph demonstrating a pretty simple principle. It applies here - notably with a caveat. First the graph.
It shows that no matter how big you make a weaker dataset, a properly designed randomized controlled trial is magnitudes better as discerning the “truth”.
Remember in this case, we have about 600 patients randomized to date on this question - maybe not exactly the question but two randomized prospective attempts. And here we attempt to over-ride those two answers with a small highly selected cohort using an “individual patient data” meta-analysis taken from previously performed randomized trials. So yes, this current dataset likely lies in between the two curves above (ie better than EHR and inferior to a single RCT) but the point remains: Taking a huge group and then performing massive selection doesn’t make it randomized strength data.
This is now the second (my opinion) rather poor application from the ICECaP dataset. Further, it is the second time when the conclusion are far broader than I believe the data supports.
Here is the former weak example from this dataset - I wrote it up back in June: scroll to “In Science, details matter” and read my commentary but it is same dataset, again with large issues where there in that prior instance, there is no context for how ADT affects PSA kinetics following radiation - a gross error - yet a major presentation at ASCO.
Prostate Cancer: We begin the dive into higher risk disease
In Science, details matter...
And yet, just this past month at ASCO, one large talk had slides that completely ignored this reality. It is, to me, a massive error in logic.
Note: in both cases, what I see is a narrative more in favor of systemic treatment. Just something to write down for the next study from this group. The narrative appears strong in this one.
In the Weeds: Assessment of the Literature
Today, we were in the weeds admittedly. I wrote it because I worry that critically evaluating medical literature is a skill that is becoming increasingly rare so I like to review how I approach things - It may not be perfect, but it is better than skimming headlines and abstracts.
In my assessment, too rarely do people track down references. We have too many authors and there is far too much pressure to publish far too many studies in my view. A strong understanding of math and statistics often seems lacking for many reading the literature. And the review process, in places, seems broken. We are becoming a generation of headlines and that is not good for medicine.
Thanks for following along as I occasionally go down rabbit holes. I do think this broader topic is critical in today’s world so it will resurface from time to time. Think of this as simply my small attempt to readjust our heading when I think I see things off course.
And if this author of one is wrong, please comment or let me know. I do this work trying to get better and will make errors or miss things - have to.
In Closing:
I like to reach out to a few leaders in the field while writing these articles and get outside input. This week in the email, I wrote something like, “I’d be hesitant to intensify without more data. What are your thoughts?” I had written the above Summary on my own well in advance of any reply, and then I got this reply:
We would have been panned if we had sent in something like this! I wouldn't read too much into it (or change anything in practice)
So it seems there are at least two of us who land on the other side on this study. You should read and think and decide where you land.
Oh well, all streaks come to an end. Thanks for the ongoing support as we try to search for a path towards better in a sea of information. Next week we’ll follow today’s trend a little further and see how the NY Times has covered radiation. Hint, much like we often present our own work, it is certainly not biased in our own favor.
ADDITIONAL REFERENCES:
EHRs and RCTs: Outcome Prediction vs. Optimal Treatment Selection








In my practice I have 1 patient in the past 5 years.
This paper may have more to do with an agenda to use Lutetium PSMA. Maybe more patients will receive docetaxel then be eligible for Pluvicto before the PSMAfore trial allows for us to use it before a taxane.