How do we value our own technology?
Did you know? Two randomized trials in 2023 demonstrate benefit to higher levels of technology. Do we argue for the value or discount it?
Way back in July :) I wrote this regarding randomized proton vs. photon trials:
But if trials land like the esophageal data and do demonstrate benefit in toxicity reduction - say with head and neck studies both in the US and Europe landing in favor of protons, then we have a different issue. Overnight, we will have real issues with lack of access to treatment demonstrated to be beneficial. Even one trial in a broad patient cohort immediately results in far too little access.
It was in this editorial piece where I argued that randomized data is absolutely required for proton therapy. But the discussion attempted to be a broader in its context arguing that as we perform trials looking at technology - ie MRI Linacs or Proton Therapy or even Cyberknife - there is real risk if the technology really begins to show benefit.
I argued then and now that we stand on the precipice of demonstrating that technology does in fact matter. Ironically, I thought it would come first in head and neck cancer within the proton trials but it is landing from MRI linacs and Cyberknife via tumor tracking / imaging - from all places, within the treatment of prostate cancer.
This year, I think there are two pretty clear examples of the machine making a difference in randomized prospective data:
The MIRAGE Trial
PACE-B data
Pause and just read that again: Two trials - each randomized prospective studies showing that all machines might not be the same.
A Quick Refresher:
In the MIRAGE: GU and GI toxicity was reduced in the MRI Linac arm.
The incidence of acute grade 2 or greater GU toxic effects was significantly lower with MRI vs CT guidance (24.4% [95% CI, 15.4%-35.4%] vs 43.4% [95% CI, 32.1%-55.3%]; P = .01), as was the incidence of acute grade 2 or greater gastrointestinal toxic effects (0.0% [95% CI, 0.0%-4.6%] vs 10.5% [95% CI, 4.7%-19.7%]; P = .003).
And now we see it in PACE-B (beyond the headlines):
Below is the late bladder GU toxicity in the PACE-B. Don’t look at the captured red box - look down at SBRT modality (CL) with a hazard rate of 2.3 and a p value of 0.002.
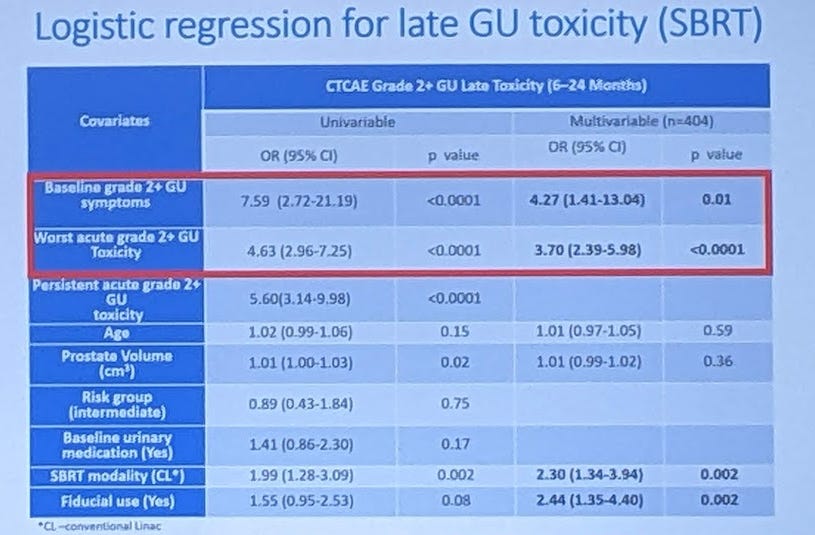
The hazard rate for late GU toxicity was 2.3x if patients were treated in a Linac based program compared to a Cyberknife program.
As I’ve pointed out, much of our data (until this trial) for disease beyond low risk is Cyberknife data. In fact, so much so, that the initial PACE-B design ONLY allowed Cyberknife machines to enroll. And then here, in really our first big look at comparing the two modalities in a randomized setting, we continue to see difference.
Consider, just perhaps, it is valid to state that the path to SBRT is easier / safer with the more advanced technology. It is literally the same findings as in the MIRAGE Trial. Hmmm… maybe the technology helps.
So the question becomes, how do we argue for the value of the technology or do we dismiss the difference?
My concern is, on issues both large and small, we often, at least from a clinical perspective, seem to dismiss it.
Let’s look at what we do: Today and Tomorrow
Today, we’ll look at how we assess our own value. How should we as a field coalesce around certain priorities that are required to allow for a clear path of innovation within our field. I think this is always an important topic, but more pertinent today on many levels as we attempt to restructure and stabilize our billing / revenue model.
1) Specific Machine Treatment Technologies
How best, as a specialty, should we argue for the value of the next generation of machines or will we be dismissive of the value that technology adds to our patients?
We started with trials and data from randomized prospective datasets. In each of these two cases, the counter argument is quite simple: it isn’t the technology, it is simply the margins.
Yet, in each trial the scientists evaluating and using the technology used the more advanced technology to treat with smaller margins. In the MIRAGE trial this was clear from the basic structure, but it is also quite clear from interviews with Dr. Kishan. According to him, the ONLY reason he was comfortable in the margin reduction was directly related to the technology. He used data and a lot of pre-study work to determine margins and then moved forward with his assessment of that data. And his assessment was the MRI linac helped to safely shrink margins.
So you are disagreeing, not with me, but with him.
And PACE-B appears to be very similar. We do NOT have granular data yet on the margins both in the linac arm and in the Cyberknife arm, but Sunnybrook out of Toronto was a linac enrollment site and here is what the lead of that program posted (I would bet he is pretty well informed as to the details - maybe not to the spreadsheet level, but it would be unwise to dismiss this commentary):
Again the same counter argument resurfaces. It’s just margins. Nothing to do with tech.
And really, if you don’t have the highest dollar capital equipment, that is pretty convenient. You get to keep doing what you are doing and try to transition your margins and your practice without asking the difficult questions. Will your results EVER be published? In most cases, no. (at least for the vast majority of those centers attempting this type of translation). So you hold onto your market share, you convince yourself that your stuff is good enough and you roll on. Often you are likely correct, but always? - I’m less convinced.
Even when it shows up in randomized data, do you think a SINGLE patient will be referred outside of a hospital system to a Cyberknife facility based on the data above (HR 2.3 late toxicity, p=0.002)? I’d place money on no. My point being this data will be dismissed in our clinics.
Is it right? I have no idea what “right” is. We all do this type of translating between trials and clinical implementation all the time. But, here, now with two sets of randomized prospective data and as our specialty presents a new billing model, I think it is reasonable to ask the question again, especially as we argue for our value within the framework of changing payment models.
Granted, if you have great experience without those tools can you offset that benefit or minimize the difference? Quite likely - or at least much of it. In fact, I argued that simply due to the contributions of Sunnybrook that PACE B would put to rest the arguments in favor of Cyberknife. And even with that experience lining up on the linac side, we still seem to see a difference. As we reviewed last week, I’m not perfect. I missed on the results and I missed on this prediction as well, but at least my context of our history allowed me to pick up this fact in the 20 seconds the slide was up.
So that is where we begin. Large machine differences. MRI linac vs. not an MRI linac. Protons vs. photons. And next we’ll make the difference more subtle. After all probably 90% (complete guestimate) feel confident that “their” machine is just as good or really close to as good for nearly all cases - especially those working within large systems quite far from a single room rural setting with physics coverage 1 day per week. How many patients have you referred beyond your own system to facilities based on a different machine technology? I bet it is very few.
I’m in the 10% that is far less certain - hence moving across the country mid-career. And yes, I’ve made referrals to MRI linacs from a proton center and referrals from a proton center for lung SBRT elsewhere due to motion management issues. Granted not often, but I try and these are real examples.
And remember, that the REAL underlying question for today is, how do we address this issue with respect to assessing our own payment structure and value within oncology? I’m telling you, it gets murky so lets keep going.
2) Adaptive treatments, multiple SBRT treatments, retreatment cases
So let’s make it more difficult to answer by asking the next question - what about adaptive treatments or multiple SBRT treatments? Certainly we can do those items easier today than in the past. Even cases that were “palliative” in the past are very complex today as we push for better. Some now are highly intensive approaches using SBRT to multiple sites with multiple isocenters. Treatments only made possible by the technology in our clinics within the last 10 years.
These approaches are multiplying in the clinic on a regular basis. Literally the majority of the “machine” “sales” talk on the vendor floor was related to adaptive treatments - where there is no clear / unified approach for payment - at least that I have seen.
Or consider retreatment cases? Remember back in the day when the answer was one of two options: nope or give “just a little” dose.
Today, we require really time-consuming re-creations of dose and we don’t de-escalate. Far from it. We’ll utilize our technology to shrink volumes and push dose higher - well above what used to consider safe or sane. This is a growing part of any practice that has newer technology within their clinics.
But consider the basic question: How will we continue to argue for that value? It is a question that is pertinent both within a fee for service model and within a bundled payment episode framework. What are the highest priority items that we should address?
While much of today’s clinical work is modeled with ROCR, what are our specialties’ priorities for growth to account for future changes? I’d like to see us have conversations to create a clear and protected innovation path.
Today’s article might have less answers than questions but sometimes that is reality. My context is pretty simple, I believe we are a technology driven field. As I’ve said, until I have x-ray vision and shoot radiation from my finger-tips, I rely on great technology.
3) Software and AI tools
Attempting to blur the line even more look at our software - the AI contouring tools or the grab a slider on the DVH and pull and it re-calcs. Deformable fusions with deformable dose - these are value add tools. They require knowledge and staffing and they allow us to unlock clinical value. And, yes, they require additional capital to purchase and significant capital from manufactures to develop.
Do you remember the days of “come into clinic - hit a button, let the IMRT optimization run for hours, maybe even leaving the clinic to come back later only to watch it fall apart when you moved from an ideal fluence to segmented leaves so it was deliverable?” - I do. It would fall apart terribly and you’d start again, and after 2-3 days you settle on something “good enough”. An “plan iteration” might take a day and eventually it was about good as it could get. Similar process today but 3 days might be 15-20 minutes - that is a value add to our patients - and it required large capital investment.
Today we are, more and more often, recontouring while the patient is on the table. If not truly adaptive, how many times do you perform QA CTs to make sure the dose is accurate over the treatment course and push out a new customized three dimensional IMRT composite plan to for the patient within one or two days based on centimeter or millimeter changes. And that is a ton of work that includes the summation of multiple dose sets, extra safety checks, extra calculations - and all of that is REQUIRED because after all, it is radiation being given to a cancer patient. And because, we do really good work giving higher and higher doses which, ironically, cuts reimbursement.
At a minimum, this work improves the safety of our treatments. And on some real level, it is this technology advancement that allows for our ability to reduce fractions. But is it well represented in our billing approach? From my perspective, many of these steps are currently not represented on any real level today - government moves slower than industry - so we try to fit new work into older codes.
From my perspective innovation is more than just machines and in places, it is more subtle. As clinicians, it is easy to often discount the progress and the value of the technology that we have seen added to our toolbox. Some are terribly capital intensive like protons or MRI Linacs and therefore quite obvious. But others are very personnel intensive requiring more staff and more safety oversight. Some require top tier servers and internet connections yet taken together, I believe they represent technologic progress and tremendous value to the treatment of cancer patients.
Should treating 7-10 metastatic lesions with SBRT - an amazingly resource intensive process that carries quite a bit of regulatory risks in most states due to its single fraction approach be bundled with a palliative 30Gy / 10 fraction bone met treatment? And multi-isocenter palliative treatments are growing rapidly with good strong level 1 data supporting the value this approach adds.
Or take whole brain vs. hippocampal sparing IMRT? Again, a new approach with proven benefits for patients. Seriously? These approaches are vastly different on so many levels. And yes, part of that difference is represented within the IMRT code, but I don’t think it is all represented. I saw the recent UT Southwestern accomplishment of taking an MRI for planning and getting a hippocampal sparing case done in about 30 minutes from a patient perspective. Amazing. And so one might de-value the work if based on simply time from that viewpoint.
But in the pictures I also counted 10 staff. 10 staff. And yes over years that will come down, but in the years until that process reaches the masses, we need dollars that account for the extra staffing that is required in addition to the capex computer technology required for such approach. And how should we account for the month or more of work behind the scenes to make that advance feasible and safe which ultimately saves dollars in the US healthcare system within our current FFS model. Have you seen Dr. Henke describe the “Herculean lift” regarding adaptive planning on an MRI Linac? That effort mandates reimbursement on some level.
Ultimately it is important as clinicians and scientists that we see the value in our field so that we can unify our voice - a requirement in such a small specialty. Me? I’m pretty simple. I think our field deserves value for nearly all of them - many of which we are compensated for today, but many of which we are not. I believe new tools and approaches will help us to push forward in achieving superior patient outcomes. To me, these tools cumulatively create our value in oncology.
So I’m now about 2 1/2 months into my more serious consideration of how the proposed episode based payment plan might land and what its effects might be. I do believe that alongside that effort we should work to clearly define an innovation path regardless of whether the ROCR model becomes reality. It’s very likely happening on some level but beyond the discussions I’ve seen.
In fact, the issues I’ve discussed above have historically been framed within a treatment “complexity” discussion or a “tumor stage” discussion - often making the debate into “our hospital treats these complex cases” while “yours does not” so we need more payment. Rather than this type of approach, I think they are more accurately and positively framed as protecting a path for innovation. Regardless of what you call it, it is vital for our specialty to protect and ensure this path exists - in both large and small centers.
Here are some ideas where I think we need good discussion:
Adaptive platforms (including auto-contour / fusions / deformable packages)
”Definitive” Re-treatment
Lv1 SABR indications perhaps including concurrent immunotherapy
Multiple isocenter treatment deliveries
Direct disease tracking in contrast to surface imaging - be that CT,MRI, fids, or PET based.
Proton therapy
I do think current utilization rates for these events today are largely modeled in ROCR. (Granted some lie beyond the ROCR model like protons with existing CPT codes and other like adaptive lie outside the model with a less well defined path - no clear CPT valuation). In the dozen or so people who have modeled their practices that I have talked to, each seems to land favorably as the ROCR rates as being balanced and representative of practice expense / reimbursement relative to current rates. But modeled today doesn’t mean modeled to support capex investment in our field over the next decade - and the two are not the same.
Is this “path” best represented via a number of smaller separate codes as is typically the case today or, at the other most extreme case, should it be a single “innovation” code that covers multiple indications so that our efforts to acquire payment are more unified across our small specialty? As I said, I don’t have all the answers, but I think it is a good time to ask questions.
I believe that market pressures related to our advances are real. Either we obtain appropriate pricing for the value we bring to oncology or there will be consolidation strictly via current market forces applied to our specialty. Let’s hope it is a combination of the two - I think that is the optimistic path.
We create value in an incredibly technology driven field. Almost across the board I believe we provide more value than pharma in the oncology space and yet 340B (while litigated and contested) exists. And yet, to me, in places, we seem dismissive of the technology we utilize for the outstanding value we create. And it is tough to argue for payment value if we, the clinicians, aren’t consistent in recognizing that value.
Radiation isn’t getting simpler for many of these of uses we discussed above - it is more capital intensive and more resource intensive - it takes a greater number of clinical partners to execute at the highest level and we need to be advocating for the value we provide.
In closing, I’d like to see more conversations to better formalize our path to protect innovation. I think creating clear paths to address issues with access to care, distribution of services, and innovation are critical to its success. Today I’ve covered many examples that are clearly documentable / verifiable along the innovation path: deformed dose plans, cumulative dose plans, multiple isocenter plans, adaptive plans, tumor tracking, fusions, AI approaches and pathways for the successful models that include SBRT platforms, MRI linacs and protons.
If it helps get reimbursement, call these the “drugs” we use.
If I have missed something or you can strengthen the list of priorities, please comment or reach out.
A “what if” story….
Consider the Cyberknife platform. If you look at the data and which programs created the data for 5 fraction SBRT treatment for prostate cancer. If you consider the volume of data created with that specific machine and the timing of those programs and then pair that with the current data demonstrating less late toxicity EVEN TODAY, it seems quite clear that - at a minimum - that machine helped to allow our field to make the progress in shortening treatments and adding value within radiation for prostate cancer.
If you think that - at least on some level - it helped us to get where we are, then understand one fundamental difference for that platform. It had a G billing code for robotic linac based surgery which provides additional reimbursement for the use of that platform. It makes sense in that the machine tracked the tumor during treatment and this was complex - significantly slowing throughput on the machine but enabling (supported by the data above) the potential for better treatment. It didn’t try to find value in existing codes, it obtained a G HCPCS code (G0339). And, to me with the benefit of hindsight, the machine appears to have added value to our field. A success story.
Now consider ViewRay - a platform that has randomized data demonstrating benefit and was generating, rather rapidly, additional data in different sites often supporting those trials to move our specialty forward without achieving payment for the platform. As we all know today, it did not end well. While much of that lies in the overarching business strategy and simple bad luck timing, part lies in funding or lack thereof. Now consider our current approach for adaptive treatments more generally as that approach is being aggressively advertised as the future.
I entered the field over 25 years ago due to its emphasis on technology. I see us as great innovators. I think that is demonstrated in a nearly 96% cure rate within intermediate risk prostate cancer using non-invasive approaches. In fighting pressures that look like the commoditization of our field, we need to continue to reach for a path to promote innovation.
As I’ve said, it is an interesting time to be within our specialty - pressured from both inside and out.
In closing consider how different the US pharmaceutical market would be if new drugs received no different up front pricing when they came to market. No extra payment, but rather they were forced into an existing cubbyhole of a generic drug pricing.
Paxlovid: ~$20 to make - pricing at $2500 / course.
(things move fast, I’ve seen this move to down to around $1300 in 48 hrs, but point remains)
Yep, pretty sure it would be different.
Thanks for following along as we search for better. Hopefully some of the points here can make us consider and evaluate the path to a brighter future.