Protons vs. Photons: Esophageal Cancer
In Part 2 of this series, we'll look at the data for esophageal cancer
First off: Happy Mother’s Day!! A Cheer for Moms!!
Last week, we looked at the possible data for benefit in a reduction in acute toxicity for lung cancer. Again, this is a personal project I’m working on looking at acute toxicity and reviewing the data. These are essentially my notes and my interpretation of the existing literature. A review of one. Whether my assessment will be right or wrong, time and future data will tell.
Esophagus, like lung, has randomized prospective data. Again it is out of MD Anderson.
Esophageal Randomized Phase IIB trial: Protons vs. Photons
This one was published in JCO in 2020 and was authored by Steven Lin, who in my opinion, has really done tremendous work in figuring out where differences in toxicity might lie between protons and photons.
The rationale for the trial was this: IMRT has shown lower cardiopulmonary doses than 3D. This is based on a single institution retrospective trial and a SEER database review, showing improvements with IMRT related to this dosimetry improvement. I found NO randomized data in esophageal cancer supporting IMRT - just a reminder that what we often accept for support for “standard of care” varies significantly by indication and is often viewed through a personal perspective of “do you have it available”, but I digress. Back to the trial…
First off, we’ll look at dosimetry differences. Not because they prove clinical differences in outcomes, but because if there is no dosimetric advantage to protons, there will be no clinical improvement in outcomes.

Again, this dosimetry data is based on 80% of this proton trial being treated with passive scanning. Timing wise it began 3 yrs later than the start of the lung randomized trial - this one started in 2012.
So from the above table, the primary places to look for toxicity improvements are from differences in:
Lung dose - V5, V20, and mean lung dose all significantly less.
Heart dose - proton mean heart dose was 57% of the IMRT dose.
Liver dose - proton mean liver dose was 19% of the IMRT dose.
(After completing two of these reviews, I’d just comment that I expect these dose differences to be most apparent in lymphopenia risk. Perhaps heart can translate cardiac events, but that will likely be beyond any acute window in all likelihood.)
The Approach was very different from the lung trial
In the lung trial, they picked radiation pneumonitis as the singular toxicity to follow. Here they took nearly an opposite approach using a total toxicity burden.
Specifically, here are the included toxicities with the assigned weightings.

Whether you like this approach or not, the list of toxicities seems like they would all matter to clinicians. I can see valid debate over the inclusion of the lowest (10-20 point weighted toxicities), but they generally make sense. Exactly how they should be weighted is a matter that will never be agreed upon, but to me, these don’t seem unreasonable.
And what did the trial show?
IMRT had 2.3x the total toxicity burden of protons. In the post-operative setting, IMRT had 7.6x the total toxicity burden of protons with an average hospital stay that was 5 days longer (13 vs. 8). It was 99.9% likely that protons achieve less toxicity than IMRT for the treatment of esophageal cancer. Early stopping rule applied. (PFS and OS were the same in the trial).
Ok. So for an esophageal patient, lets use the 2.3x global figure. Can we translate Total Toxicity Burden in CTCAE Grade 3 or higher toxicity rate? Why that specific question? Because that is the question I’m trying to answer. :)
I think it is quite difficult to translate total toxicity burden in CTCAE Grade 3 or higher toxicity without the actual database and another review of the charts. But looking through the list, they are ALL lung or heart toxicities and so if you can document Grade 3 toxicity rate for esophageal cancer, you can expect a 50%-55% reduction in toxicity moving to proton therapy. Well… maybe…
This table lists out the differences for the non-operative complications. As you can see, most “events” were mild. Most pericardial effusions were asymptomatic, most pleural effusions were asymptomatic, and most radiation pneumonitis was Grade 1 or 2 with only a single Gr3 event.
So me, guestimating, I’d cut the 55% in half and say maybe a 25% reduction in Grade 3 toxicity - it might be more or less of a reduction - I don’t really know looking in from outside the database.
Note: in the Baumann trial the reduction was 27% to 11% (that retrospective comparison study had 55 proton and 95 IMRT esophageal patients out of 1483 total patients). This prospective data, including my fudge factor for Total toxicity burden to CTCAE Gr3 or higher toxicity would move 27% with IMRT to ~20% (maybe 22% or 23% - lots of Grade 1 toxicities - I doubt a bigger difference) with protons. Neither the single institution retrospective data nor the SEER IMRT vs. 3D data attempted to parse Grade 3 or higher toxicity rates so these don’t hold good data for us.
Looks similar to the lung data
Again, I like broad patterns and through two sites with two sets of randomized data, I see a trend that looks like there are very subtle differences in Gr2 and Gr3 toxicities - that if you are really precise, you can measure. If you measure or look for the wrong thing, you miss.
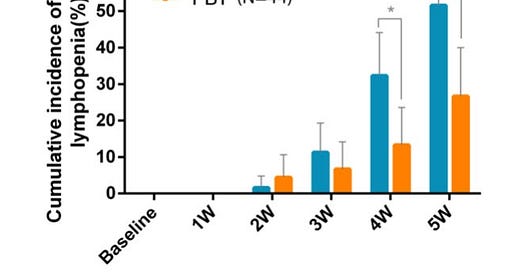
And once again, in this dataset, lymphopenia showed up - just like it did in the lung cancer data.
This is Grade 4!! lymphopenia (<50 cells / uL, Grade 3 is <500 cells / uL). Basically a 50% reduction from 50% to 25% with the use of passive scanning proton therapy. This is exactly what the lung data shows. Small differences in Grade 2 or lower toxicities reveal themselves in large differences in Grade 3 and here, Grade 4 lymphopenia toxicity. The low dose bath to the heart, lungs, and liver impact lymphocyte counts significantly.
Clearly the randomized study is clearly the strongest data. But here is a rather thorough look at the literature. I think it is worth mentioning next.
Efficacy and Safety in Proton Therapy and Photon Therapy for Patients With Esophageal CancerA Meta-Analysis - August 2023.
This is a meta-analysis of 45 studies. It is really a pretty good review of our literature. Kudos to the authors. Dosimetry wise, it shows improvements in lung doses, cardiac doses, cardiac substructure dosing and 3 studies that demonstrate less bone marrow dose.
Specifically, on the question of acute CTCAE toxicity, this meta-analysis found:
The incidence of grade 2 or higher radiation esophagitis was 50%, grade 2 or higher radiation pneumonitis was 2%, grade 2 or higher pleural effusion was 4%, grade 2 or higher pericardial effusion was 3%, grade 3 or higher radiation esophagitis was 8%, and grade 4 or higher lymphocytopenia was 17%.
Proton therapy was associated with significantly decreased grade 2 or higher radiation pneumonitis and pericardial effusion, and grade 4 or higher lymphocytopenia.
Overall, both acute and late toxic effects were relatively low, except for grade 2 or higher radiation esophagitis, grade 2 or higher pericardial effusion, and grade 4 or higher lymphocytopenia.
So again, the differences they found were with Gr2 or higher esophagitis and pericardial effusions with lymphopenia being the ONLY metric different at a Grade 3 or higher level.
Like the IMRT vs. 3D SEER data, this meta-analysis shows improvement in overall survival with proton therapy compared to IMRT. (Just noting strength of data comparisons.)
So there you have it. Based on a meta-analysis proton therapy improves overall survival compared to IMRT - top of the pyramid data (so the experts say - sarcasm). Me, I’ll stay in the crazy camp and say randomized prospective trials are top of the pyramid.
And 4 more to round out the work:
Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced esophageal squamous cell carcinoma - February 2018.
A dosimetric comparison between proton beam therapy (PBT) and photon radiation therapy, showing that PBT significantly reduces the dose to organs at risk, such as the lung and heart, which correlates with lower toxicity but with a caveat:
Regarding the correlation between the grades of toxicities and the dosimetric parameters, no significant correlation was seen between the occurrence of grade 2 pericardial effusion and the dose to the heart.
To me, once again pointing to need to really be precise and specific to quantify any differences - they will be quite subtle and similar to the lung randomized trial. If not asked “just right” it will be easy to miss any potential difference - at least that is what I see in the data.
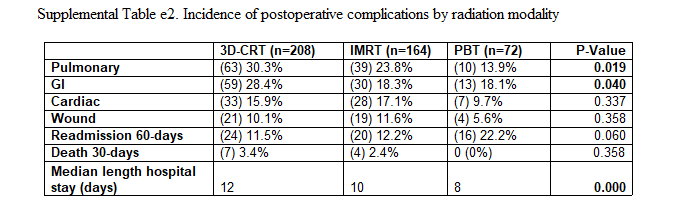
Predictors of Postoperative Complications After Trimodality Therapy for Esophageal Cancer - August 2013.
Another paper by Steven Lin out of MD Anderson (he’s done a ton of work in this area - kudos!!). This looks at 444 pts (3D - 208; IMRT - 164; PBT - 72) and describes the CTCAE toxicity events. This data represents only passive scanning proton therapy.
The most frequent postoperative complications after trimodality therapy were pulmonary (25%) and GI (23%).
Mean lung radiation dose (MLD) was strongly associated with pulmonary complications, and the differences in toxicities seen for the radiation modalities could be fully accounted for by the MLD delivered by each of the modalities.
The Supplemental data contains the following breakdowns for toxicity by modality - this is the multi-variate analysis. These are only described as “perioperative complications” - I could not find exact definitions or a reference to CTCAE level.
Finally, two studies from Japan:
Preliminary treatment results of proton beam therapy with chemoradiotherapy for stage I–III esophageal cancer - January 2016.
This is a retrospective group of 47 patients treated with photons initially with a proton boost. There is no comparative attempt, but they document the following rates of CTCAE toxicity. It serves to show some baseline CTCAE expectations for combination treatment - a “blurred” line average perhaps.
With respect to grade 3–4 late toxicities, there were no pleural or pericardial effusions, but two patients (4.3%) had esophageal stenosis, one patient (2.1%) had fistula, and two patients (4.3%) developed radiation pneumonitis.
Point: Any event rate will be very low for Grade 3 or higher toxicities - likely with either approach.
Clinical Results of Proton Beam Therapy for Esophageal Cancer: Multicenter Retrospective Study in Japan - July 2019.
This is a retrospective study of 202 patients treated with proton therapy for esophageal cancer.
There were two patients with grade three pericardial effusion (1%) and a patient with grade three pneumonia (0.5%). No grade 4 or higher cardiopulmonary toxicities were observed (Common Terminology Criteria for Adverse Events version 4.0).
Here is the full toxicity table:
Again, we see pretty large numbers for Grade 2 toxicities that really become far less frequent in the Grade 3 or higher group. I think that becomes the important message - high grade events become pretty uncommon.
Summary:
That is my look into the data on the esophageal cancer side for acute differences between photons and protons. The data is dominated by the randomized prospective data that is 80% passive scanning. There is very little pencil beam data demonstrating clinical outcomes that I found.
Once again, it seems quite similar to the lung data - we see far more Gr2 toxicities that seem to manifest more clearly in higher grades of lymphopenia. In that specific toxicity (which is related to the low dose bath difference in integral dose) a difference between the two arms seems more clearly defined in both the lung and esophageal data.
Total toxicity burden or a broad approach of Grade 3 toxicity that captures hospitalizations for any cause seem to be more robust in their ability to discern differences in the two modalities. Lymphopenia appears in both sites (lung and esophagus) as the single best CTCAE Grade 3 category to differentiate outcomes.
A Note on the Importance of Radiation Induced Lymphopenia:
Radiation induced lymphopenia is a relatively well known predictor of survival. Here are four representative studies indicating its importance and illustrating our ability to model risk for its development with treatment:
56 studies - 13223 patients - 11 cancer types. Most use lymphocytes<500/ul with a reporting interval of 2-3 months following treatment. Using this approach radiation induced lymphopenia results in a hazard rate for worse overall survival of 1.7.
Radiotherapy-Related Lymphopenia Affects Overall Survival in Patients With Lung Cancer
901 patients - Grade 3 or higher lymphopenia. They studied lung cancer patients to develop a model and tested it on 305 patients with esophageal cancer.
Here are the curves for the studied lung cohort and the model applied to an esophageal cohort:

915 patients: 303 with breast cancer, 612 with intrathoracic tumors including 291 esophageal cancers. Below is the hazard rate for those with lymphopenia (<500/ul) compared to no lymphopenia (>1000/ul).
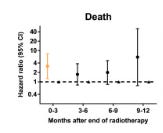
Finally, even in a site like breast cancer, we see this association / linkage between radiation induced lymphopenia and survival in this study from China, the University of Maryland and Case Western.
735 patients - mean peripheral lymphocyte counts were 1580/ul prior to treatment and 990 /ul following treatment. At the end of radiation, 60.5% had lymphopenia. RapidArc, mean lung dose and chemotherapy were risk factors for lymphopenia and this study and the prior one, both found low dose volumes to be specifically linked to the risk of lymphopenia.
(Note: The low dose concept with lung v5 resurfaces quite consistently within this specific subset of our literature. In most places, we are arguing v5 doesn’t matter and that plans are better if we dismiss it and focus on higher dose metrics, but here, within this little corner of our data, it shows up. To me, it speaks to caution being warranted in thinking this area is completely understood.)
That’s what I found within our literature looking at our expectations for improvements in acute toxicity reduction if we move from IMRT to protons in this setting.
Thanks for reading along as I create my own personal reference document. With Head and Neck prospective data on the way this year, I’m not going to address that site. Instead, we’ll keep moving inferiorly as we continue the project. And from now on, the data will be weaker as there is NO randomized prospective data below the diaphragm - really amazing / sad to type that over 30 years into clinical roll-out. (but maybe we can just use systemic analysis instead - sarcasm and a topic for another day. Until then keep pushing for better and have a great week).