Protons 101: Two new technology trials are on the horizon in radiation oncology for pancreas cancer.
Does radiation really have a role in the management of advanced pancreatic cancer and how should technology trials be structured and judged?
Omissions or errors: I’m an author of one so, if I missed something or left out something important, please comment and I’ll do some work and clean anything up.
MRI Linac has two new pancreatic trials which look to push for better outcomes in that disease.
As we prepare for the OPC Trial comparing protons to IMRT in oropharynx cancer (ref 1), we’ll need to review benchmarks for what will be / should be defined as a winning study in the landscape of a radiation oncology technology trial.
In the MRI Linac world, there are two pancreas studies being launched with a focus on technology in our field. One is less adventurous, while the other appears quite bold.
ViewRay is listed as the sponsor in both and kudos to them and the clinical teams for moving forward with these trials in the field of radiation oncology. As I’ve said since moving to Oklahoma City for proton therapy.
Until I have x-ray vision and shoot radiation out of my fingertips, we are a technology based field of medicine.
There is no way around that fact and as a field we should embrace technology and work hard to prove its benefits.
Study 1: SMART trial
It’s a phase II single arm trial with primary endpoints of Gr3 or higher toxicity at 90 days for patients with borderline resectable or inoperable pancreatic cancer (ref 2). Planning to enroll 133 participants with dose to deliver of 50 Gy in 5 fractions delivered twice a week. Real-time imaging and adaptive planning will be used.
So top tier treatment in a phase II trial. It would have more potential impact if we didn’t have the second trial. But we have the second one and so this trial becomes unlikely to move the needle. Maybe some confirmatory items but realistically, the next trial seems to set the bar.
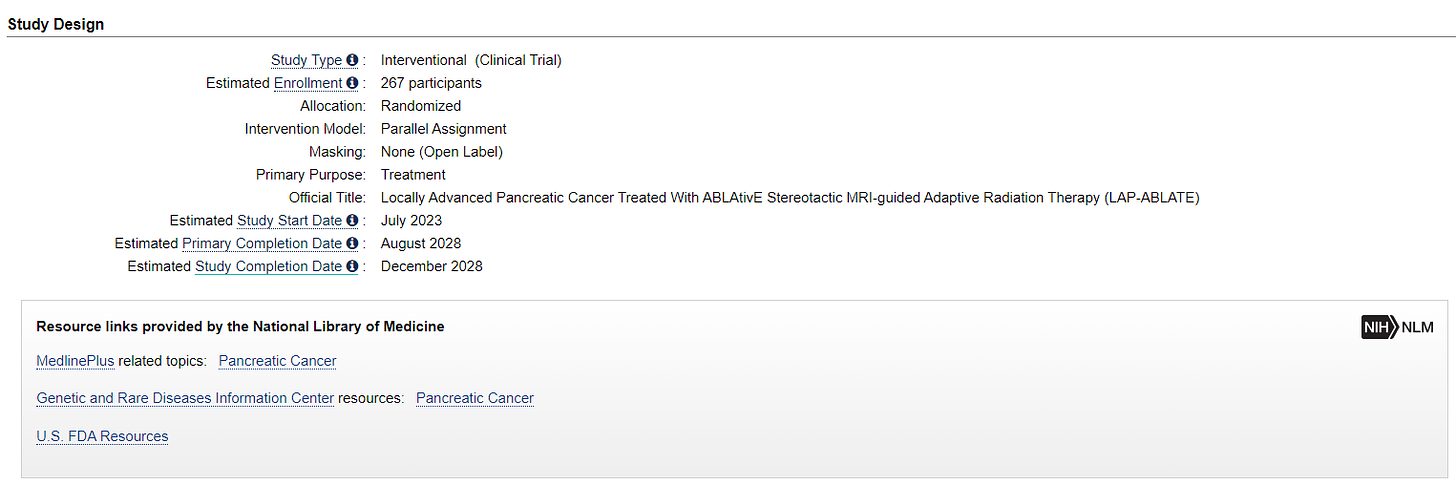
Study 2: LAP-ABLATE
This is a prospective randomized controlled trial (2:1) comparing induction chemotherapy followed by ablative Stereotactic MR-guided on-table Adaptive Radiation Therapy (SMART) vs. chemotherapy alone in locally advanced pancreatic cancer. (Chemo is Minimum 8 cycles of FOLFIRINOX OR 12 doses of gemcitabine/nab - paclitaxel and radiation is MRIdian SMART 50 Gy in 5 fractions. (ref 3)
267 participants - so about 179 in the SBRT arm.
The primary object is simply:
OS at 2 years. Kaplan-Meier survival curve.
There are 7 secondary objectives outlined.
Progression-Free Survival (PFS) [ Time Frame: 2-years ]
Local Control (LC) [ Time Frame: 2-years ]
Regional Control (RC) [ Time Frame: 2-years ]
Distant Metastasis Free Survival (DMFS) [ Time Frame: 2-years ]
Patient Reported Quality of Life (QoL) using EORTC QLQ-C30 questionnaire [ Time Frame: 3-months, 12-months, 24-months }
Patient Reported Quality of Life (QoL) using EORTC QLQ-PAN26 questionnaire [ Time Frame: 3-months, 12-months, 24-months ]
Treatment-related toxicity [ Time Frame: 90 days ]
Simply stated from my perspective, the LAP-ABLATE trial is BOLD. It takes a machine and platform that today has tremendous upside and asks a simple question - does it help improve survival compared to just chemotherapy?
It is so simple and straightforward that it carries pretty significant risk. And to me, if either positive or negative, it will cause a lot of issues for our field. In that regard it is similar to how I view the OPC proton trial - both are pivotal trials for a segment of our subspecialty.
The trial isn’t perfect, but nothing is. Typical for today, both arms include chemotherapy. As a radiation oncologist, I’d like to see the SBRT combo arm split into just ablative SBRT dose or the combination arm but again, can’t do everything, and this trial deserves a lot of credit for its straightforward and aggressive search for better.
Discussion: Where will these trials take us for borderline resectable or inoperable pancreatic cancer?
Today, I would say that the strongest retrospective data and best clinical use case for the MRIdian System is in the upper abdomen. (ref 4, 5)
(Note: I don’t have clinical use experience. I have talked with experts and know the technology reasonably well but I do not have hands-on clinical experience. I’ve discussed at length the MIRAGE trial (ref 6) and while it is important, it simply holds a much smaller clinical opportunity for benefit).
The upper abdomen is a straightforward example case. Makes an easy pitch. Bowel is sensitive, the adaptive treatment to adjust dosing seems critical, and imaging during treatment likewise seems to provide significant value. Results with traditional treatment are often quite poor today for this type of cancer and there appears to be massive room for improvement.
Especially in the LAP-ABLATE trial, this priority use case is up for study with a straightforward outcome metric of overall survival. It is, frankly, what the proton trials haven’t been able to do near enough of.
This trial is also interesting as it asks a basic radiation question at the same time - does radiation add benefit to chemotherapy for these cancers. Realistically if this trial fails, I don’t know that radiation oncology will have a use case in the upper abdomen for these advanced pancreatic cases.
If it is negative for OS, PFS, and DMFS, then one really can’t argue that SBRT or even more broadly radiation should be utilized at all - this will have real time imaging and adaptive - it will be as good as you can get. You can’t argue for protons over this in this region of the body or any other technology approach - certainly not ungated, unimaged traditional SBRT. I guess if LRC, and PFS / DMFS are in favor of SBRT there might be an argument that with longer term follow-up, OS might become significant, but there isn’t much wiggle room in this one.
And that is why it is a great trial. Because science and treatment wise, this appears to help define the best path forward. Let’s be clear, I don’t care one bit whether radiation is needed at some site - I work at this point in my career because I want to - what we we want is the best treatment with the best outcomes. But frankly it is not very often these days that you see such a straightforward trial design. By comparison, one should read and study the evolution of the OPC proton therapy vs. IMRT trial. The primary endpoint is PFS with secondary endpoints of toxicity. And worse, it is a “non-inferiority” trial (< that will haunt it)
I encourage you to read the OPC reference: It clearly describes how the NRG seemingly pushed the non-inferiority decision. And I think that story gives great insight into why we see designs like LAP-ABLATE so infrequently in America.
But if LAP-ABLATE is positive.
But if it is positive, do you believe that physicians running programs without these types of live plan adjustments and on-table imaging will increase their referrals to the nearest MRIrdian machine while decreasing their patient numbers? Or do you think that they will simply do “their” version of something as similar as they can with their equipment.
The answer is the later.
Ideally that shouldn’t happen. As then the scientific question becomes: would that “close enough” version pass in a prospective trial like this? That question becomes unanswered. But this is what I strongly believe will happen. This approach in our field happened FAR less 2 decades ago from my perspective. It is a fundamental weakness of radiation oncology in the US today.
Here are 2 examples for this assessment of how the US healthcare system functions.
First, the MRI MIRAGE Trial (ref 7):
Despite really clear explanations by the primary investigator as to the rationale for the margin reduction being very strongly tied to the improvement in technology, there is a vocal argument that this is more simply a margin reduction trial . The PI argues the 2 mm reduction is specifically tied real-time imaging to account for motion and direct contouring of target without an intermediary fusion between CT and MRI. (ref 8 - recommended podcast episode).
But if you admit it is technology - ie the MRIdian machine, then you are admitting that non-real time imaged linac SBRT treatments cause more acute toxicity. And so, my opinion, it is far more convenient to “soften” the answer to “margin reduction” which can be more widely implemented without admitting the difference in approaches.
Second, the DARS trial example:
Three years ago, the DARS trial prospectively proved that using a specific approach and forcefully lowering doses to the pharyngeal constrictors that patient outcomes improved. (ref 9) (This is important. It wasn’t added as an objective and pushed, it is a different approach with constrictors playing a dominant role upfront in planning being prioritized over low dose regions).
To follow this trial, entities would simply have a change in internal planning methods. And even in this case, the ONLY prospective OAR dosing ever shown in radiation oncology (correct me in the comments if you know of another), most people I ask “do it their way.”
And the reality of that type of approach is that we will never know if that is good enough - not without a trial. Heck, I saw a recent “AI” paper out of MDACC and Stanford published in 2023 looking at planning optimization and it DID NOT include the pharyngeal constrictors on any level. (ref 10) Again this is arguably our strongest OAR dosimetry metric in our entire field of medicine as it is the only one proven prospectively.
This change requires no capital investment and can be mirrored on many levels with relative ease, yet instead many choose to soften and expand their approach and we, as a subspecialty, continue forward with less pronounced shifts.
In closing: It is an interesting time to be in radiation oncology. I think it is likely a matter of time before we determine, prospectively, that not everyone has the same equipment and that the equipment directly relates to outcomes.
I don’t know if it will be here, or with a proton trial, or with some other machine, but at some point, it will happen. And to me, this reality is consistent with the NRG, from an outsider viewing perspective, softening the OPC trial design. Protons can’t really be “THE standard” in OPC cancer. We need to have the line blurred and the requirement of a non-inferiority trial makes that a nearly guaranteed reality.
Instead of that type of approach, we need strong trial design with clear answers that impact change. And alongside of that initiative, we need to develop better methods in our healthcare system to utilize and share the highest cost capital equipment more efficiently.
Based on what I see today, I say “Kudos!” to the teams and leaders of these two trials and to ViewRay for the support to push for better with a technology trials for our field and our patients.
Thanks for reading!
REFRENCES
OPC Trial The Journey from Concept to Activation:
https://www.sciencedirect.com/science/article/pii/S1053429617301170?via%3DihubStereotactic MRI-guided On-table Adaptive Radiation Therapy (SMART) for Locally Advanced Pancreatic Cancer
https://clinicaltrials.gov/ct2/show/NCT03621644Locally Advanced Pancreatic Cancer Treated With ABLAtivE Stereotactic MRI-guided Adaptive Radiation Therapy (LAP-ABLATE)
https://clinicaltrials.gov/ct2/show/NCT05585554Wash U Phase I data for pancreatic cancer
https://pubmed.ncbi.nlm.nih.gov/35878713/French Prospective Registry experience
https://www.frontiersin.org/articles/10.3389/fonc.2022.842402/fullProtons 101: Was the MIRAGE MRI Prostate Cancer Trial Positive?
MRI MIRAGE Trial:
https://jamanetwork.com/journals/jamaoncology/fullarticle/2800541The Accelerators Podcast: MIRAGE discussion https://accelerators.buzzsprout.com/1839275/12125004
DARS Phase III study of dysphagia-optimized intensity modulated radiotherapy
https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.6508Deep Learning AI Head and Neck Plan QA
https://www.practicalradonc.org/article/S1879-8500(22)00388-5/fulltext#%20
www.protons101.com, home of the original Protons 101 website.
Content for the Protons101 blog written by Mark Storey MD.
Updated 2/7/2023 to reflect changes in RADCOMP expectations.