Prostate Cancer Radiotherapy - Can We De-Escalate "Better"? (REFERENCE SERIES)
A review of external beam NCCN standards (non-SBRT) and my perspective that data supports less ADT use in UIR when dose escalation is utilized.
Perhaps we can do better than just dropping fractions and adding more systemic medications. Maybe improvements in technology now allow a different paradigm, especially in unfavorable intermediate risk disease.
Disclaimer: Author of one, please comment or reach out if I’m missing important data. A rather lengthy article but serves as my history, a reference type document for current treatment standards and rationale to eliminate ADT in most unfavorable risk disease. So we cover a lot - 24 references. Even more important post following NEJM on active surveillance. (ref 25)
Prostate Cancer: A look back and perhaps a vision for the future
A few weeks ago I wrote on how I believe our specialty can increase our impact on cancer care moving forward (Protons 101: A path to greater impact - refocus on cancer outcomes and embrace technology). A renewed focus on improving outcomes is critical. Shorter and about the same isn’t good enough. In a second article I tried to explain why I don’t really like trials that simply move to less fractions (Protons 101: Non-inferiority Trial Design).
Since writing those articles, I got to thinking about prostate cancer and our current path of de-escalation. Typically it involves simply reducing the number of fractions and keeping our results - hopefully - non-inferior to longer treatment courses.
But as I dug back through the reference articles and my own little piece of history, I got sidetracked a bit. Today is a look back at my perspective which is quite different from consensus. It ended far broader than I anticipated at the onset but hopefully is more entertaining than NCCN guidelines. It covers a number of trials to give flavor and substantiate, at least in my opinion, why we should be able to improve our path for many patients moving forward.
“My” first randomized trial: 78 Gy vs 70 Gy
LOL - it was Zagars’ and Pollack’s trial - they designed it (ref 1). I was a resident and Alan simply gave me the opportunity to help write up the initial toxicity release (ref 2). Few people have published one standard of care defining randomized trial, but Alan has published three pivotal trials in radiation oncology - impressive by any standards. A sincere thank you to these two physicians.
Times were different - half the patients had Gleason 6 or lower disease. 2/3rds with PSAs<10. In the end, FFF (freedom from failure) was 85% vs. 78% at 5 yrs, 78% vs. 59% at 8 yrs, and then 73% vs. 50% at 10 yrs with 2x prostate cancer deaths in the lower dose arm. GI toxicity was higher - essentially double, but then our work (which he allowed me to publish as a resident) helped show the importance of rectal dose constraints.
On a secondary analysis, for PSA’s less than 10 (not really a clean match to current stratifications) FFF was 78% vs. 66% and the curves didn’t separate until about yr 7 and the p value based on shape of curves and patient numbers didn’t meet significance.
When we discussed the 5 yr trial results (can’t speak to the 10yr - I was gone), Alan thought this difference between low and intermediate risk outcomes was important. At that time, pushing the dose higher resulted in significantly more GI toxicity. I think that was very reasonable, especially back then.
We didn’t know if the rectal data metrics we created would be robust and reproducible. The benefit of control leading to prostate cancer survival benefit was almost certainly higher in higher risk patients. And finally, this was a single institution approach with nuance in technique (for example block edge was AT GTV for the boost posteriorly to minimize rectal dose - yep « that is correct - no CTV, no PTV, no 5mm to block - block edge at prostate), so recommendations were written up conservatively.
But in the end, the trial as designed simply shows benefit to higher dose with a MASSIVE improvement in outcomes (23% different in disease control) between 70Gy and 78Gy with the increase in failures resulting in more prostate cancer related deaths.
The MDACC experience replicated
RTOG 0126 and GETUG 06:
These are two similar designs comparing 70.2 Gy to 79.2 Gy (ref 3) and 70 Gy to 80 Gy (ref 4). Patient populations were simply different back then. But here are the relapse free results.
RTOG (5 yr): 60% vs. 75% for the low / high dose arms.
GETUG (5 yr): 61% vs. 72% for the low / high dose arms.
RTOG fell to 53% and 69% by 8 yrs. In the RTOG, similar to the MDACC data, you see again a trend towards something more than just local control issues - the distant metastatic rate was higher in the low dose arm.
I’ll summarize these three randomized dose escalation trials simply: dose escalation improves bDFS. And now two trials point to improved outcomes beyond just PSA - prostate cancer specific survival in the MDACC trial and distant metastatic rate in the RTOG trial.
And so we move forward: More dose is better. Higher risk, but better at curing the cancer:
Times were different. These were mainly 3D CRT trials and dose escalation carried real risk of higher toxicity. Less was established on rectal and bladder constraints and the long term toxicity risks of dose escalation. Plus, at the time, there seemingly was far greater concern about moving from large centers to nationwide implementation which tended to result in a conservative approach in recommending technique and fractionation changes.
So we had two options, further dose escalation or adding in ADT with more modest dose escalation. Due to toxicity concerns, we opted for the later adding in ADT quite often as we knew that failure rates of at least 15% and likely, in a broader national setting, closer to 25%-30% at 5 yrs were less than ideal.
Today’s “Standard”: Guideline therapy
NCCN is interesting (ref 5). It arguably is THE reference document. Here are quotes in italics taken directly from the current NCCN Prostate cancer guidelines:
Kuban and colleagues published an analysis of their dose-escalation trial of 301 patients with stage T1b to T3 prostate cancer. Freedom from biochemical or clinical recurrence was higher in the group randomized to 78 Gy compared to 70 Gy (78% vs. 59%, P = .004) at a median follow-up of 8.7 years. The difference was even greater among patients with diagnostic PSA >10 ng/mL (78% vs. 39%, P = .001). A longer follow-up (mean 14.3 years) found that improvements in biochemical and clinical recurrences were sustained, with lower rates of additional cancer treatment and better prostate cancers specific mortality.
An analysis of the National Cancer Database found that dose escalation (75.6–90 Gy) resulted in a dose-dependent improvement in OS for patients with intermediate- or high-risk prostate cancer. In light of these findings, the conventional 70 Gy dose is no longer considered adequate.
A dose of 75.6 to 79.2 Gy in conventional fractions to the prostate (with or without seminal vesicles) is appropriate for patients with low-risk cancers.
The NRG Oncology/RTOG 0126 randomized clinical trial compared 79.2 Gy (44 fractions) and 70.2 Gy (39 fractions), both in 1.8 Gy fractions, in 1499 patients with intermediate-risk prostate cancer. After a median follow-up of 8.4 years, the escalated dose reduced biochemical recurrences, but increased late toxicity and had no effect on OS.
Patients with intermediate-risk and high-risk disease should receive doses of up to 81.0 Gy.
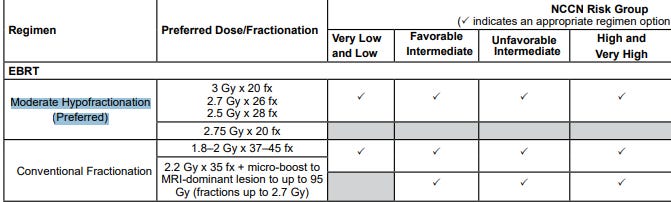
My summary of the NCCN statements above:
Low Risk Disease REQUIRES 75.6 - 79.2 Gy.
Intermediate and High Risk dose should be a step above your Low Risk Dose.
But really, you can do almost anything and shorter is “preferred”
Hypofractionation Standard Approaches:
60 Gy / 20 fractions:
Two trials: CHHiP (ref 6) and the PROFIT (ref 7) studies. CHHiP is cited 2x which is backwards to me when I look at how they were structured.
PROFIT - clean simple - no ADT: 60 Gy / 20 fxs vs. 78 Gy / 39 fxs
Intermediate Risk 85% DFS
CHHiP used a lower dose standard arm - lower than current NCCN recommendations of 74 Gy / 37 fxs and then used ADT in nearly all patients (~97% usage despite 15% LR patients and only 13% HR). The trial included a third arm at 57 Gy / 19 fxs which proved inferior.
5 yr DFS: 88.3 (74), 90.6 (60), 85.9 (57)
Together they are quite interesting. I know you have to be careful comparing different trials, but basically the ADT addition bought 5% fewer recurrence and / or 5% less dose. Look at the data. 60 alone, 57+ADT, or 78 Gy are all ~85%.
70 Gy in 28 fractions:
Two additional trials. Back in the day, when we were running the trial at MDACC, Cleveland Clinic was going the hypofractionated route (ref 8, 9 - 5yr, 10 yr). RTOG 0415 then looked at this dosing scheme on a national level (ref 10).
First the RCT data from RTOG - Low risk disease 73.8 Gy vs. 70 Gy. Patient population was ALL Gleason 6 or lower and PSA<10 (1 in 5 had a PSA<4 with Gleason 6 disease) and no ADT allowed.
For low risk patients at 5 yrs, DFS rates of 85.3% (standard) and 86.3% (hypofractionated) were achieved. Both late GI and GU toxicities were increased with the shorter course of treatment.
Interesting to note in this study Phoenix definition picked up about 1/2 of the recurrences. I think this begins to illustrate that, depending on your imaging approaches and how quick one is to start ADT, you can potentially halve or double your recurrence rate in prostate cancer if using Phoenix definition and that effect is likely higher today with PSMA scans. Remember this in any radiation prostate trial you read using Phoenix definition.
The series out of Cleveland clinic was a single institution series and the precursor to this national trial. It was not a prospective trial dataset so a clear step down in strength but it is a good dataset. 60% of men in this series received ADT with a median duration of 6 months.
Results: The overall 5-year ASTRO biochemical relapse-free survival rate was 82%. The 5-year nadir + 2 ng/mL rate for patients with low-, intermediate-, and high-risk disease was 94%, 83%, and 72%, respectively.
The 70 Gy data, at least to my eyes, appears no stronger than 60 Gy. LR at 85% and IR at 83% with 60% ADT utilization. It continues to support these types of doses in LR and likely FIR cases. EQD2 and BED calcs would make one think it is a bit higher, but I tend to look at outcomes over those calcs.
70.2 Gy in 26 fractions:
And then Pollack moved to Miami and ran another randomized dose trial:
70.2 / 26 fractions vs 76 Gy in 38 fractions enrolling 303 men (ref 11). ADT was used in high risk, and for some intermediate risk (likely for more than one intermediate risk factor based on my work with him and quite common at the time). At an alpha / beta of 1.5, this is about 5% hotter than 70 / 28.
Globally the trial achieved 5 yr bDFS of 79% in the conventional arm and 77% in the hypofractionated arm.
More specifically, 80% bDFS in intermediate without ADT with conventional and 82% in the hypo arm. It bumped up to 85% in intermediate with ADT and 70%-75% in the high risk groups.
My take on these three approaches:
They are all amazingly consistent datasets. Broadly for intermediate disease: 80% or so control with no ADT. 85% with some and 90% if you treat all comers down into low risk populations. 20 fractions is obviously the shortest and 70.2 is BED the highest from 1.2 - 1.5 alpha/beta ratios, albeit the data seems very similar across the 3.
And one additional older dataset:
MSKCC: As MDACC was winding up its randomized trial and Cleveland Clinic was pursuing hypofractionated treatments. MSKCC came just a bit later with IMRT and pushed doses higher - significantly higher (ref 12).
Here are details from the long-term publication (with one typo corrected for them :)):
Between August 1997 and December 2008, 1002 patients were treated to a dose of 86.4 Gy using a 5-7 field IMRT technique… A total of 587 patients (59%) were treated with neoadjuvant and concurrent androgen deprivation therapy.
For low-, intermediate-, and high-risk groups, 7-year biochemical relapse-free survival outcomes were 98.8%, 85.6%, and 67.9%, respectively (P<.001), and distant metastasis-free survival rates were 99.4%, 94.1%, and 82.0% (P<.001), respectively. No prostate cancer-related deaths were observed in the low-risk group. The 7-year prostate cancer-specific mortality (PCSM) rates, using competing risk analysis for intermediate- and high-risk groups, were 3.3% and 8.1%, respectively (P=.008).
I still think disease then vs. now is a bit different with Gleason scoring changes and stage migration issues, but at 7 yrs they hit 85.6% and at 5 yrs ~88% for intermediate disease. I have this trial mentally ear-marked as the best IMRT outcomes: ~88% at 5 yrs.
Issues are obviously single center, non-randomized. Pretty similar to the Cleveland Clinic data and the University of Florida Proton data we will review below.
The other interesting historical piece to me is that this approach gained very little traction nationally. I don’t remember this dose ever recommended in NCCN. Never the standard arm for an inferiority trial. Yet, the BEST historical results published with IMRT and for some reason, very little traction.
I assume it had to be toxicity concern on some level. I’m not saying this trial didn’t show what it shows, but on some level, I assume someone was concerned about risk on a broader rollout. Like I said before, it was a different time and we worried more about certain techniques - that if rolled out more broadly, had nuance that perhaps might not translate.
(if you know better why, please comment or reach out)
Again, this data is consistent. Here we add more dose and with moderate ADT usage, we bump bDFS up towards 90%. This is now ~81 Gy at 2 Gy per fraction and it achieved a long-term prostate cancer mortality risk of 3% in the intermediate risk group.
My argument is simply this. We have room to improve. Anything shorter that was compared to traditional fractionation cannot be expected to have outcomes any better than 80%-85% (the above data covers 11 trials - mostly prospective data). And depending upon your patient population, you’ll likely need significant ADT to get that result. If you want to do these types of approaches and want to get closer 90%, you need to treatment almost every intermediate risk patient with ADT.
That is my read of where we have been. Now to where we are today.
What are potentially better options?
We have three strong published examples that certainly appear to demonstrate that we can do MUCH better today with LRC. I’ll focus outside of SBRT to keep concepts simpler (I’ll likely return to SBRT in the near future). A proton series, FLAME SIB trial, and two brachy trials.
The Florida Proton Experience:
It is non-randomized and will clearly bring the most questioning because, well, it is protons. But it is consecutive patients enrolled in a prospective outcome tracking trial. If anything one step stronger than the Cleveland Clinic and the Memorial Sloan Kettering data referenced above as it prospectively enrolled. If you believe those, you should at least consider this strongly.
70 Gy / 28 fractions for LR and 72.5 Gy / 29 fractions for IR (ref 13). Modern series so much less stage migration issues. Unlike the above studies, ADT was generally NOT used. Only 21 of 582 men received ADT.
Here are the 5 and 7 yr results:
LR 98.8% / 98.8% - ADT 1%
FIR 97.2% / 95.2% - ADT 1.9%
UIR 93.1% / 88.8% - ADT 11.5%
The 5yr Intermediate Risk outcomes are at nearly 95%. Or take the 7yr 92% mark. This is FAR BETTER than anything we have reviewed with external treatment and ADT usage was minimal - much less than any trial approaching these bDFS marks.
Why are these results so strong even compared to single center non-randomized data with such minor dose escalation? It doesn’t make sense?
I’m not certain either and that argument, one that simply says it doesn’t quite match history, to me, is as strong as any. Run studies 100 times and you will see outliers. Such is life. But beyond chance, here are my thoughts after 4 years with protons for why it might be slightly more effective dose to target:
In passive scanning, there is nearly NO tradeoff on dose to prostate / margin coverage around the regions of the neurovascular bundles. With IMRT, this region IS the decision point - narrower lowers rectal dose but is tighter. With passive scanning, you get this big rectangular dose box - it is different (ex shown below). (I actually worry about this in new centers starting with PBS).
SV dose is higher at 60 Gy prior to the reduction. Perhaps this is a bit better.
Base dose is higher: ~5% - should contribute some.
BED being 1.1 might be off another 5%
Some other low alpha / beta particle effect that is poorly defined today.
From my experience, this is the approach I try to mirror and I think I’m in this ballpark of these results - as I relayed earlier, I track all my proton pts in my own spreadsheet I update at least every few days. 4 years in and I trust this approach FAR more than SBRT 38-40 Gy / 5 fraction uniform dosing with respect to PSA response, PSA nadir, and toxicity. Simply, it works.
(currently my UIR pre-tx PSA 9.3: median PSA 0.65 at 1yr with no ADT - should decrease with additional follow-up looking at sheet. Ultimately pretty confident based on these kinetics they will do well at or above 90% at 5 yrs.)
(I’m not crazy - protons are not magical but to quickly dismiss this trial which my 4 yr experience seems to replicate is quite arrogant. And the 90% mark has been beaten in proton publications at least two additional times. By UPenn at 4 yrs with UIR at 93% (ref 14) and with 8yr LR/FIR data at 92% (ref 15). And then a third time out of Japan at 91% bDFS for intermediate and 86% for high risk (ref 16). So it isn’t one off data. I still think the COMPARRE trial will be negative - they choose same dosing per arm at just 60Gy and more importantly the time is far too early).
But I get it - until there is randomized data, it will be strongly questioned (earliest option I’d guess is a 10yr data from COMPARRE before there is any chance). But don’t fear, we have other great approaches.
Two randomized prospective treatments clearly improve outcomes for tumor control relative to HIGHER doses than were used in the above non-inferiority trials.
FLAME Randomized Phase III Trial
This trial out of the Netherlands (treatment 2009-2015, published 2021) (ref 17) looks at intermediate and high risk patients and seriously ups the dose. The standard arm is 77 Gy at 2.2 Gy / fraction and the focal boost arm performs SIB to 95 Gy at 2.7 Gy / fraction to the visible lesion. OARs over the SIB boost but still… a far cry from 74 Gy / 37 fractions.
Standard arm is around 81-83 Gy (calc’d EQD2 81.8 Gy) and boost arm is well over 110 Gy (calc’d EQD2 115.8 Gy) to the visible lesion. Note, more modern and alpha / beta calc in this trial is based on 1.2.
Patient population: 84% HR, 15% IR, mean PSA 15.2. Despite 84% having high risk disease, only 65% of patients in the trial were treated with ADT - Kudos to the treating physicians!! Better treatments / Less ADT.
At 5 yrs, the bDFS was 92% in the SIB arm and 85% in the “standard” arm. Toxicities were statistically the same - maybe a slight bump in Gr2 toxicity but no difference. There was no difference in distant metastatic free survival in this trial evaluating two very high dose recommendations.
So backing up - 75% for HR in the 70 Gy / 28 fx Cleveland Clinic data vs. 92% in the SIB arm here. The failure rate for the 70 Gy / 28 fraction approach is over 3x higher! And ADT is about the same or even less in FLAME. Yes with time results often trend higher due to better metastatic workup / stage migration issues, but this is beyond that. I firmly believe it illustrates higher radiation dose can significantly improve the cure rate.
I think the only concern here would be a general fear of the dose levels - at least in the US, we haven’t gone this high and even the standard arm would make people loose some sleep, but it is clear that for some intermediate and high risk patients, we need to be pushing higher.
And finally two brachy boost trials:
Your pick - the UK HDR brachy trial (ref 18) or the ASCENDE RT trial (ref 19).
I’ll spend less time and quickly summarize these important trials that utilize brachy to boost the disease. But it illustrates the point, we need MORE dose than we thought in the past.
In the UK HDR brachy boost trial, brachy resulted in a 31% reduction in the risk of recurrence. In the ASCENDE-RT trial adding brachy instead of 78 Gy moved the b-PFS rate from 84% to 89% at 5 yrs. At 7yrs the difference increased to 75% to 86% and at 9 years the difference was a massive 83% compared to 62%.
In one trial we eliminated a full 1/3rd of recurrences and in the other over half of the failures were avoided with a brachy boost.
Of course, there is nothing magical about brachy. Oh other than it puts the most dose in the cancer and the least in other places. It is the “protons” to “protons” so to speak.
So back to the NCCN guidelines: Version 1.2023 - 9/16/2022
What is preferred for intermediate and high dose?
3 Gy x 20 fx
2.7 Gy x 26 fx
2.5 Gy x 28 fx
I literally have no idea why those are preferred over the FLAME trial results. One can choose not to include brachy or protons - that makes sense. Until our national leadership figures out billing basics and lets us treat external in one facility and move to a nearby surgery center for brachy, we can’t be implementing the RO-APM and then forcing brachy via a guideline. And protons clearly need RCT data to be considered as preferred. No doubt.
But FLAME?? What is the excuse there? We just like more failures? We don’t like so much dose? We want to keep the importance / impotence of ADT by moderating radiation dose? (couldn’t help myself)

A quick detour - Why ADT in UIR?
Just so you know I’m not making this part up, I’ll quote directly from the NCCN guidelines:
The addition of short-term ADT to radiation improved OS and cancer specific survival in three randomized trials containing 20% to 60% of patients with intermediate-risk prostate cancer (Trans Tasman Radiation Oncology Group [TROG] 9601 (ref 20), Dana Farber Cancer Institute [DFCI] 95096 (ref 21), and Radiation Therapy Oncology Group [RTOG] 9408 (ref 22).
So the data is ALL based on trials starting well before 2000 and all treat to clearly inferior doses. One trial, the Dana Farber trial treats to 70 Gy and the others treat to 66 Gy. The TROG trial is still behind paywall - haha. So can’t get numbers - it is a hazard rate instead. But the others, reduce disease specific mortality to 4% and 3% with ADT. My guess is something similar beyond the paywall.
And then you have a secondary analysis of the RTOG data referenced later in the paragraph.
The RTOG 9408 Secondary Analysis
This secondary analysis showed benefit in the UIR reducing distant metastatic rate and disease specific mortality (ref 23). Presented as hazard rates in the abstract but about a 10% difference in distant metastatic rates at 10 yrs and nearly 2x that difference in prostate cancer specific mortality. (surprises me that deaths are far more than the metastatic rate difference but didn’t dig too deep.)
(added via addendum a new reference I think is reasonable to include, but it doesn’t change anything significantly - in fact it generally reinforces my arguments - ref 26).
And a second second look: MSKCC data revisited
This looks back at the MSKCC data - picking the patients over >81Gy and parsing by FIR / UIR criteria. Adding hormones reduced DM rate and PCSM rate in UIR. But despite the dose being closer (81 at 1.8 is 76Gy EQD2 and 85Gy is 79.7Gy), the control rate was poor in the series at 65% at 8 yrs.
Control for this dose is relatively poor but this was when IMRT was young. I know they worried, like we did at MDACC, about rectal toxicity I just forget the nuances of these patients or I’d put it in print. Imaging was different, setups were different, optimization was different and the dose isn’t really that high by todays standards. I think this dose would achieve better control today - no doubt.
An editorial comment: Times were different. I added this trial as it maybe a large part of the rationale for us stopping to push dose higher and to move towards ADT. It would be quite ironic if the very best IMRT results ended up being responsible for us stopping to push on dose and overusing ADT because we thought 76 or 80 Gy (with early IMRT likely to iso in a homogeneous plan) was top end of what we could do.
My take:
I don’t like making any decisions on such old data. I tend to believe the PREMISE of the conclusions more than the data. And yes there are little other pieces of old data, but wow. These old trials are why tons of men nationally get ADT for UIR - straight from NCCN and their chosen references.
Again, the premise I believe:
If we don’t push dose and can’t achieve good control rates, can ADT help decrease distant disease and prostate cancer specific mortality? Sure. And it is more obvious in higher risk disease? Sure.
My counter argument is simply this. If you get bDFS up around 90% with radiation alone, the opportunity for ADT to help important metrics like DM rate or disease specific survival either vanishes completely or is really very small. Comparatively, if 40% or 50% of men fail, there are plenty of events to warrant ADT.
Doc: So for your intermediate risk cancer, I’m recommending 20 treatments and 2 anti-testosterone shots.
Patient: “So why do I need to take those terrible hormones doc?”
Back some about 20 to even 30 yrs ago, when Gleason scores of 4 and 5 (total scores) existed and we often treated with a 4 field box to 66Gy or maybe 70Gy over 7 weeks, it appeared to help. ‘Course it was a different time where about 4 in 10 men recurred. We do a lot better today.
*noticing the concerned look and thinking a bit* Plus then more recently, we looked back at some of that old data and we tried to apply today’s fancy staging rules to it and it still looks like it probably helps. Remember your PSA is 10.4 and half the cores were positive. If we add in ADT we can get cancer free results almost as good as doing a fancy “integrated boost” like in the Netherlands or I guess even external followed by an implant.
“So why don’t we just try that? I don’t want to be neutered”
*Pause…* You know, here we really just try to do “best practices / guideline medicine” and what I’m offering is the “preferred” approach. I can show the guidelines if you want.
A Canadian study illustrates my “new paradigm” - more dose / less ADT. The study is a propensity-score model retrospective study (ref 24). But they did it and it showed that in 326 patients treated with brachy boost (i.e. more local dose), ADT didn’t help metastatic free survival.
And although the NCCN references 878 publications, there is no reference for the decision to not require ADT with a brachy boost. I assume it’s this Canadian trial or something similar - clearly not worth noting.
“Preferred” Regimens in UIR and HR:
Just to state clearly: none of the above “preferred” hypofractionation schemes have ANY comparisons to even the standard “lower dose” arm in the FLAME multi-institution prospective randomized trial (an excess of 80 Gy) which is now a proven inferior option to further dose escalation. All appear based on equivalent dose metrics and outcome metrics to be inferior with respect to delivered dose and local control. We have the MSKCC data, the FLAME data and multiple brachy trials demonstrating dose response above 78 Gy, yet we PREFER something that is very likely inferior, because it is shorter?
If you look at the decision tree for UIR disease, you see the bias. They routinely recommend ADT along with EBRT and then make it optional with brachy. Again, brachy ain’t magical, we need dose. There are 3 trials above that escalate dose to the prostate and demonstrate, what would appear to be, superior control results. Rather than the “preferred” approach being equivalent to 74Gy or 78Gy at 2 Gy per fraction, we should be encouraging higher doses and not covering up mediocre radiation results with the added toxicity of ADT. Makes no sense. Further the data supporting any real benefit in UIR is largely based on clearly inferior dosing of the primary. And I believe the data today argues that if we increase dose to the primary we can negate that need.
The Path Forward:
Clearly I don’t quite see eye to eye with the guidelines today. But here is the issue. Back in the day, as I described above, we couldn’t push dose higher. We were limited by our knowledge, planning, and delivery techniques. We very appropriately opted to moderate dose and toxicity and add in ADT.
Today, technology affords a different answer than just reducing fractions. Working on this piece and once again stepping through history, I feel pretty confident that there is a good path for us to reconsider our approach. That path is not hidden. It is the one the scientists in the Netherlands proceeded along with the FLAME trial design and execution.
And we should follow their lead. The paradigm can be shifted. We should reassess our reliance on ADT. We should be pushing for higher doses. We should streamline and simplify the NCCN guidelines to the single hypofractionated course we think is strongest today (even if that takes a big national trial). We should push doses and push to de-escalate on the ADT side of the treatment - almost certainly in UIR and likely in many HR patients.
ADT is a toxic treatment - far worse than 10 or 20 extra visits to a radiation department. Let’s get back on track, strive for the absolute best results with radiation possible and delay ADT in as many people as possible for as long as possible.
REFERENCES:
Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer
https://www.redjournal.org/article/S0360-3016(07)01173-X/fulltextComplications from radiotherapy dose escalation in prostate cancer: preliminary results of a randomized trial
https://pubmed.ncbi.nlm.nih.gov/11020558/Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer The NRG Oncology RTOG 0126 Randomized Clinical Trial
https://jamanetwork.com/journals/jamaoncology/fullarticle/267501470 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial
https://pubmed.ncbi.nlm.nih.gov/21147514/NCCN Prostate Guidelines:
https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdfConventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(16)30102-4/fulltextRandomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer
https://ascopubs.org/doi/full/10.1200/JCO.2016.71.7397Hypofractionated Intensity-Modulated Radiotherapy (70 Gy at 2.5 Gy Per Fraction) for Localized Prostate Cancer: Cleveland Clinic Experience - 5 yr
https://www.redjournal.org/article/S0360-3016(07)00535-4/fulltextTen-Year Outcomes of Moderately Hypofractionated (70 Gy in 28 fractions) Intensity Modulated Radiation Therapy for Localized Prostate Cancer
https://www.redjournal.org/article/S0360-3016(19)30182-8/fulltextNRG Oncology RTOG 0415: Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4981980/Randomized Trial of Hypofractionated External-Beam Radiotherapy for Prostate Cancer
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3805927/Long-term Survival and Toxicity in Patients Treated With High-Dose Intensity Modulated Radiation Therapy for Localized Prostate Cancer (MSKCC)
https://www.redjournal.org/article/S0360-3016(12)00679-7/fulltextFive- and seven-year outcomes for image-guided moderately accelerated hypofractionated proton therapy for prostate cancer (UFPT)
https://pubmed.ncbi.nlm.nih.gov/34965846/Four-year outcomes of hypofractionated proton therapy for localized prostate cancer. (UPenn 4yr)
https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.7_suppl.80Long-term Clinical Outcomes in Favorable Risk Prostate Cancer Patients Receiving Proton Beam Therapy (UPenn Proton long-term)
https://meridian.allenpress.com/theijpt/article/8/4/14/472107/Long-term-Clinical-Outcomes-in-Favorable-RiskLong-term outcomes in patients treated with proton therapy for localized prostate cancer (Japan Proton)
https://pubmed.ncbi.nlm.nih.gov/28879658/Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial
https://pubmed.ncbi.nlm.nih.gov/33471548/Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer
https://www.sciencedirect.com/science/article/pii/S0167814012000102Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer
https://pubmed.ncbi.nlm.nih.gov/28262473/Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial
https://pubmed.ncbi.nlm.nih.gov/21440505/Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial (Dana Farber)
https://pubmed.ncbi.nlm.nih.gov/18212313/Radiotherapy and Short-Term Androgen Deprivation for Localized Prostate Cancer - 10 yr. (RTOG 9408)
https://www.nejm.org/doi/10.1056/NEJMoa1012348?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi.nlm.nih.govEffect of Androgen Deprivation on Long-term Outcomes of Intermediate-Risk Prostate Cancer Stratified as Favorable or Unfavorable A Secondary Analysis of the RTOG 9408 Randomized Clinical Trial
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2770281Does ADT benefit unfavourable intermediate risk prostate cancer patients treated with brachytherapy boost and external beam radiotherapy? A propensity-score matched analysis
https://pubmed.ncbi.nlm.nih.gov/32619455/Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer
https://www.nejm.org/doi/full/10.1056/NEJMoa2214122#.ZA0OluODShw.twitterA New Risk Classification System for Therapeutic Decision Making with Intermediate-risk Prostate Cancer Patients Undergoing Dose-escalated External-beam Radiation Therapy
https://www.europeanurology.com/article/S0302-2838(13)00257-1/fulltext





