Review of Prospective Randomized IMRT clinical data as of 2023 (REFERENCE SERIES)
This article reviews the literature supporting IMRT being superior to 3D.
Being online, the goal is more of a living reference piece of material. As I find articles, they will be added. If you know of articles or missing studies, please reach out or comment.
Review of Utilization of Treatment Modalities in Radiation Oncology
First, I think it is important to realize the uptake and utilization of IMRT in our field. Below are national estimates from 2019.
National Utilization of IMRT vs. PBT (remainder of cases are 3D, SRS, brachy etc)
Site: IMRT Utilization, PBT Utilization:
Anal: 74.9%, 1.1%
Bladder: 42.8%, 0.2%
Breast: 6.9%, 0.2%
Cervix: 29.1%, 0.1%
CNS: 68.6%, 1.1%
Colorectal: 32.7%, 0.3%
HN: 69.8%, 0.8%
Liver: 23.1%, 1.5%
Lung: 24.1%, 0.4%
Lymphoma: 23.3%, 0.3%
Pancreatic: 48.4%, 0.8%
Prostate: 66.1%, 1.5%
Upper GI: 53.6%, 1.4%
Uterine: 24.4%, 0.2%
(Numbers via an RO-APM document - now 4 years old but likely still very representative of todays usage market - my guess is that both IMRT and PBT have increased slightly, with IMRT rising by a larger percent and PBT by a larger relative percentage gain. Mark Storey MD)
As shown IMRT has widespread adoption based largely on improvements in dosimetry. I agree with this approach. But I AM an outlier. I picked up my individual practice with 4 1/2 years of a contract remaining at pay well above the 75th percentile to move to a proton facility with limited days cash on hand at the time because I believe that dosimetry differences impact clinical outcomes. Most people don’t do such craziness. So I’m a believer in dosimetry and now, lets look at the data.
Randomized prospective trials of IMRT vs. 3D - all tumor sites.
I have not included prospective cohort studies - for example in Nasopharynx or the secondary analysis of RTOG 0617. These are strong tier 2 level studies, but here we’ll look at 6 prospective randomized trials I found. For the “best” 3 trials I found illustrating an improvement with IMRT over 3D - I added a “My Take”
Head and Neck cancer - the Xerostomia trial:
I believe this to be the most commonly quoted trial showing an advantage of IMRT (ref 2). It is a trial from India including 60 total head and neck cancer patients (28 3D and 32 IMRT) that showed a reduction in Gr2 xerostomia. No difference in recurrence rates with 10 failures in the 3D arm and 14 in the IMRT arm (opposite of an ideal answer) with 2 early salvage neck dissections in the in IMRT arm (negative for residual but not in the ideal direction). At 10 years, there are 11 deaths in the 3D arm and 16 in the IMRT arm but OS curves seem no different as shown below.
The win was in Gr2 xerostomia (mod symptoms - copious water, lubricants, moist foods). 59% vs. 89% in favor of IMRT.
Subcutaneous fibrosis did show a statistical benefit in favor of IMRT at year 1. That benefit appears graphically to remain “long-term” but lost significance beyond year 1 due, at least, in part to the small patient numbers and wide confidence intervals.
My Take:
The trial appears to show a reduction in toxicity - no difference in cancer outcomes in a very small randomized trial. Do I BELIEVE that IMRT is worlds better for head and neck cancer? YES. I would not treat a non larynx, non-unilateral case without IMRT inside a developed world healthcare market. But be clear, the randomized prospective data is very limited in scope.
Postoperative Cervical Cancer - GI toxicity reduction
The PARCER trial is another trial from India - larger, with 300 enrolled - published in 2020 (ref 3). It again shows toxicity reduction only - no benefit in cancer related outcomes.
From the abstract:
At a median follow-up of 46 (interquartile range, 20-72) months, the 3-year cumulative incidence of grade ≥ 2 late GI toxicity in the IG-IMRT and 3D-CRT arms were 21.1% versus 42.4% (hazard ratio [HR] 0.46; 95% CI, 0.29 to 0.73; P < .001). The cumulative incidence of grade ≥ 2 any late toxicity was 28.1% versus 48.9% (HR 0.50; 95% CI, 0.33 to 0.76; P < .001), respectively. Patients reported reduced diarrhea (P = .04), improved appetite (P = .008), and lesser bowel symptoms (P = .002) with IG-IMRT. The 3-year pelvic relapse-free survival and disease-free survival in the IG-IMRT versus the 3D-CRT arm were 81.8% versus 84% (HR 1.17; 95% CI, 0.68 to 1.99; P = .55) and 76.9% versus 81.2% (HR 1.03; 95% CI, 0.62 to 1.71; P = .89), respectively.
The article lies beyond a paywall for me for any further analysis.
My take:
Better patient numbers and a significant reduction in important toxicities. This trial appears to be a pretty clear win. Dosimetry differences for the two approaches are not included in the abstract, and if you look at the very next article on Cervix cancer, in that trial, there were no bowel dosimetry differences. So on some level, that tend to temper my analysis. That said, it appears to show toxicity reduction with no difference in tumor outcomes.
Cervix Cancer Definitive Treatment - “Acute toxicity”
This definitive cervix cancer trial was published in January of 2022 in Cureus (ref 4). 54 women with locally advanced cervical cancer were randomized to either 50 Gy with 3D or IMRT along with cisplatin and subsequent brachytherapy. The paper does not include a description of primary or secondary endpoints that I can find. It is presented as much as a dosimetry improvement paper as a toxicity paper. They measured CTCAE 4.0 toxicity within 90 days with a chi-square test/Fisher’s exact test to compare toxicity. For a prospective trial, the lack of clear primary and secondary endpoints and no power calculation detracts from the publication.
The results state that IMRT dosimetry was improved for bladder and femoral head dose - not for bowel or rectum. The clinical acute 90 day toxicity was reported in the abstract as:
“Patients in the 3DCRT arm had significant grade 1 and 2 anemia and neutropenia compared to the IMRT arm.”
But more precisely, on review of the results toxicity table, the ONLY difference lies in Gr1 toxicities. Gr 2 toxicities for both anemia (2 per arm) and neutropenia (1 per arm) are identical. Not so much of a win.
Breast Cancer - 3 total trials
1: KROG 15-03
KROG 15-03 is a randomized study from Korea published in 2020 looking at whether IMRT can improve dermatitis in the treatment of breast cancer (ref 5). It is the largest trial at 693 patients published in 2020. Similar to the prior trials it showed a toxicity reduction with no difference in tumor control outcomes.
The entire results section of the abstract:
Of 693 patients, 349 and 344 patients received 3D-CRT and IMRT, respectively. There was no significant difference in LRRFS between the two arms. Conformity index of planning target volume was significantly superior in the IMRT arm than the 3D-CRT arm (p < 0.001). The mean lung dose and V5-V50 for the ipsilateral lung were significantly lower in the IMRT arm than the 3D-CRT arm (all p < 0.05). The incidence of grade 2 or higher dermatitis was significantly lower in the IMRT arm (p = 0.009).
This article is again beyond paywall - it is ironic that science is now often only available with paid subscriptions. Seems like currently licensed physicians could see the “science” that shapes the field without relying upon venders for data or paying out of pocket per article. grrrr…
My take:
I’d like to dig into the techniques. Was 3D wedges or forward planned. Was the IMRT inverse or forward planned? Lung dose improvements likely have minimal clinical impact. You can cool off the skin with IMRT and that makes sense. Gr2 dermatitis is nice to minimize, but we probably negate this difference with hypofractionation, APBI for some, and forward planning MLC plans with reductions off the inframammary fold - my guess. And if you look at US utilization, breast cancer is BY FAR the lowest utilization site. So no one really believes there is benefit to IMRT over modern forward planned tangent fields for whole breast treatment.
2: APBI Pain trial
An APBI trial out of Rocky Mountain Cancer Centers with 656 patients. 38.5Gy / 10 BID treatments (ref 6). Primary endpoints of patient reported pain and cosmesis.
Pain was improved with IMRT at 2 and 3 yrs (note not different at 1, 4, and 5 yrs) and cosmesis was no different for the primary endpoint. MD cosmesis was actually statistically worse in the IMRT arm at 5 yrs. I think pretty clearly this is not an impressive result.
(Note: I didn’t know of this trial. Think of IMRT vs 3D in the APBI setting related to the Florance trial (ref 7) vs. the RAPID trial (13% to 32% late radiation toxicity) (ref 8). Perhaps it is all in fractionation and not using BID, but here we see no impact of IMRT reducing toxicity. In order of enrolled patients, RAPID easily is largest, this is second and the Florence Trial is the smallest. Perhaps we see what we want to see.)
3: LAD perfusion:
62 women with left sided disease who required IMC nodal treatment were randomized at the University of Michigan between IMRT and 3D (ref 9). It was a technology based trial and utilized DIBH for the IMRT - so the IMRT has 2 potential advantages - “IMRT” and “DIBH”. The 3D was planned and treated off a free-breathing scan.
Primary endpoint was SPECT % decline in perfusion of the heart and SPECT left lung perfusion - ie nuclear medical imaging changes due to the radiation affecting the heart and left lung.
28 IMRT and 26 3D analyzed « they lost 5??? people in the planning process due to not tolerating the sim between the two arms. (I think that speaks to trial complexity on some level - 5 of 62 not tolerating simulation is a massive percentage. 1 per ~300 would be more expected to me.)
Primary endpoint was LAD perfusion and left lung perfusion. Both showed no difference. A secondary outcome of left ventricular ejection fraction did show improvement with IMRT.
Conclusion does go on to state, “Clinical practice should recognize the importance of minimizing cardiac dose, even when already low in comparison to historical levels.” I would just say that was after prospectively answering that DIBH and IMRT together did not accomplish the primary endpoint over non breath hold 3D.
The two primary endpoints were negative. There was a secondary finding with ejection fraction. For comparison, this would be like the current RADCOMP trial only showing a partial benefit in the planned ancillary trial (ref 10), and I would therefore say, this is largely a negative trial.
Addendum: Additional studies added.
Thanks to: Ashwin Shinde MD - Vanderbilt University for helping to add to the trial list. Assessments remain mine individually (mainly stated in case I have an error).
Central Lung Palliative Study - Esophageal QOL at 2 wks.
This is a Canadian trial published in Feb 2022 where 90 patients were randomized to standard RT or esophageal-sparing intensity modulated RT (ES-IRMT - max dose (0.1cc) to the esophagus limited to 80% of RT prescription). (ref 11)
Primary outcomes was esophageal QoL at 2 wks post RT measured by FACT-E-ECS (a validated patient questionnaire for esophageal cancer patients). Patients could be treated using either a 20 Gy / 5 fraction approach or a 30 Gy / 10 fraction approach (physician discretion).
The trial cleverly required that 5 cm of esophagus needed to be treated to over 50% of dose for enrollment - so they picked people getting significant esophageal dose and then keeping the esophagus max point dose to 80% of rx even if tumor coverage was compromised. (One of the few studies I’ve seen use pre-planning metrics prior to enrollment.)
For the primary endpoint there was a trend towards benefit with IMRT with a p value of 0.06. A secondary metric of CTCAE 4.0 symptomatic esophagitis (Gr2 or higher, per article - technically NO Gr3, Gr4, or Gr5 so the difference is Gr2 toxicity) was different (p=0.002). Benefits in favor of the IMRT arm were found more in the 30Gy fractionation on a post hoc subgroup analysis. OS was unaffected.
My Take:
A strong technical writeup and trial structure. The primary endpoint trended towards a difference in showing QoL differences, but it was only a trend. There did appear to be a secondary endpoint of Gr2 toxicity reduction with IMRT. Overall, it appears to show a slight win for IMRT in a palliative setting. Certainly helpful to know for authorization if you see a large difference in your planning.
Cervical and Endometrial Cancer: Acute GI toxicity
This is a US trial published in 2019 and I missed it on searches somehow (ref 12). It reports patient reported toxicity and QoL surveys in the treatment of women with cervical and endometrial cancer.
This was a phase III multicenter randomized controlled trial stratified by dose (45 vs 50.4), chemotherapy, disease site and then randomly assigned to 3D or IMRT. No extended fields, all just pelvis. 3D could use field in field for homogeneity. For IMRT, PTV expansion was 7mm.
High quality study with all validated well described metrics. Primary end point was change in acute GI toxicity from baseline to 5 wks measured by EPIC. The primary endpoint was met (barely) with IMRT having less decline in bowel function compared to 3D at 5 weeks (p=0.048). Secondary analysis supported the primary endpoint with IMRT patients reporting less diarrhea, less fecal incontinence and needing less medication with significantly stronger p values.
CTCAE Physician reported toxicity however showed no difference. 1 Gr4 AE in the standard arm, and Gr3/4 AE rate of 16% in the IMRT group and 11% in the 3D group (so less Gr3 with 3D) and Gr2 or higher with IMRT again slightly higher at 26% vs 22%.
From the discussion and I think this is a fair assessment:
In this randomized trial, pelvic IMRT resulted in less impact on bowel function during treatment than standard pelvic RT. Similarly, treatment with IMRT resulted in less impact on urinary function during treatment. QOL metrics demonstrated that patients treated with IMRT also had a smaller decline in physical function and additional treatment-related concerns during the course of RT.
My Take:
Well done and well documented top tier data. To me the data is positive for a stepwise improvement, but at the same time, not overly impressive. The question in my mind is, I don’t believe this level of “win” would be a win in protons. CTCAE toxicity was no different. If this trial presents long term differences that persist in bowel function with longer follow-up, that would significantly add to the strength of the data. Data would seem applicable across a range of pelvic indications.
Prostate Cancer - Acute GI and GU toxicity
This is a single institution randomized prostate cancer trial out of Brazil looking at 3D vs. IMRT using 70 Gy / 25 fractions (so 2.8 Gy per fraction) published in 2016 (ref 13). It used RTOG criteria for GI and GU toxicity. 3D arm was 6 field technique. Contouring by a single physician with review by 2 others (interesting).
215 patients enrolled. Arms balanced. As expected, IMRT performed better from a dosimetry perspective. Below are the main graphics that illustrate the findings. The top graphs are acute toxicity through 6 months and below are Kaplan-Meier curves of late Gr2 or higher toxicity.


No obvious faults and the results arguably are as strong and pertinent to real outcome differences as any trial in the list. 5 yr phoenix definition DFS of ~95% in both arms.
My Take:
Results seem reasonable and probably this trial, more than any other, might be the strongest. It was published well after the US moved to IMRT for prostate cancer as standard, but it really seems straightforward and well done. I’m not sure why I haven’t seen it referenced previously. And I like the dose fractionation approach.
Head and Neck cancer - 2 additional Xerostomia trials
The PARSPORT trial is a phase III randomized prospective trial comparing 3D to parotid sparing IMRT from 2003 to 2007 (ref 14). UK trial with lower doses of 60 to 65 Gy in 30 fractions - surprising definitive dose that I think most would clearly state as too low especially in light of some of the recent failed de-escalation trials even in HPV+ disease. That said, I don’t think dose necessarily detracts from the premise of looking at reducing parotid dose as validation of IMRT.
94 total patients enrolled and 34 evaluable in the 3D arm and 39 in the IMRT arm - so again, quite small patients numbers. Primary endpoint was Gr2 or worse xerostomia (LENT SOMA).
Results show a signficant reduction in Gr2 or worse RTOG salivary gland side effects and less xerostomia.
There was no change in tumor outcomes, with the IMRT Kaplan Meier curve statistically equal but running below 3D for local regional progression-free survival.
My Take:
More data that demonstrates reductions in dry mouth if parotid dosing can be reduced. IMRT certainly accomplishes this better than 3D treatment. Interestingly for the period of the study, there is no mention of nausea as a toxicity between 3D and IMRT which often was reported around that period as worse with the jump to IMRT (proposed due to posterior fossa dose).
This trial is the 3rd prospective trial to compare IMRT to 2DRT (their term) in nasopharyngeal head and neck cancer to evaluate xerostomia. Primary endpoint was severe xerostomia using RTOG criteria at 1 yr. The study is out of China and enrolled 60 patients from 2001 to 2003. Patients were treated to 66 Gy in 33 fractions in both arms. There were patients that received a brachy boost - couldn’t find numbers - probably muddies the water a bit but not much.
Parotid dose fell from 61.5 Gy to 32.2 Gy with the move to IMRT. Primary endpoint of Gr2 or higher toxicity at 1 yr was 82% vs 39% with a highly significant p value. Secondary metrics of parotid function favored IMRT as well.
My Take:
Around 60 patient is and was the magical number back in the day. There are 3 total trials demonstrating benefit to IMRT with improvements in less xerostomia compared to IMRT all right around this size. All three are consistent with highly significant improvements in demonstrating less toxicity. Despite low numbers of patients, the consistency of the 3 trials makes this a strong area of our data demonstrating IMRT is superior to 3D for head and neck cancer.
In Closing:
That is a summary of what I could find that represents the collection of IMRT prospective data in our field. While I’m sure I’ve missed trials, let’s just say, our data is rather limited. Nothing shows a tumor outcome advantage and I find sparse US, Canadian, or Western European data (three total trials to date). Further some of the trials you might classify as wins are demonstrate quite minor improvements in low grade toxicities.
For a subspecialty that, at times, strives to be so very based in structure and “data driven”, one must look back and say we have done quite poorly in providing clear science to support the large scale transition to IMRT (approximately reimbursed at 2x 3D). Yet, without data but with time, it has become the dominant modality for 5 of 14 sites with greater than 20% utilization for every site but breast.
Clinically, in my perspective, IMRT is indicated across a far broader swath of patients than it has been proven. It is “better” in most definitive cases. I believe that. ‘Course, I believe in protons.
Note: I have not said the bar height should be identical for protons - future discussions for future posts. Here just establishing our current standards of care based on our past prospective work for IMRT.
REFERENCES:
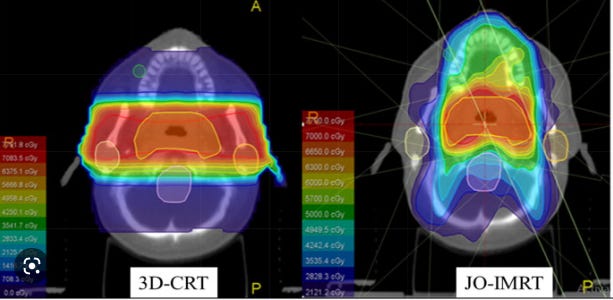
Dosimetric and radiobiological comparison in head-and-neck radiotherapy using JO-IMRT and 3D-CRT
https://www.sciencedirect.com/science/article/pii/S1319562X22002522Intensity-modulated radiation therapy versus three-dimensional conformal radiotherapy in head and neck squamous cell carcinoma: long-term and mature outcomes of a prospective randomized trial -xerostomia
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7493335/Late Toxicity After Adjuvant Conventional Radiation Versus Image-Guided Intensity-Modulated Radiotherapy for Cervical Cancer (PARCER): A Randomized Controlled Trial
https://pubmed.ncbi.nlm.nih.gov/34506246/A Prospective Randomized Study of Intensity-Modulated Radiation Therapy Versus Three-Dimensional Conformal Radiation Therapy With Concurrent Chemotherapy in Locally Advanced Carcinoma Cervix
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8818092/Postoperative radiotherapy with intensity-modulated radiation therapy versus 3-dimensional conformal radiotherapy in early breast cancer: A randomized clinical trial of KROG 15-03 - dermatitis
https://www.sciencedirect.com/science/article/abs/pii/S0167814020308185A prospective Phase III trial evaluating patient self-reported pain and cosmesis in accelerated partial breast irradiation utilizing 3-D versus intensity-modulated radiotherapy
https://onlinelibrary.wiley.com/doi/10.1002/cam4.4242External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)32515-2/fulltextAccelerated Partial-Breast Irradiation Compared With Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial
https://pubmed.ncbi.nlm.nih.gov/32840419/A Randomized Comparison of Radiation Therapy Techniques in the Management of Node-Positive Breast Cancer: Primary Outcomes Analysis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6938718/Cardiotoxicity in Breast Cancer Patients Treated With Proton or Photon Radiotherapy: A RadComp Ancillary Study
https://clinicaltrials.gov/ct2/show/NCT04361240Palliative Radiation for Advanced Central Lung Tumors With Intentional Avoidance of the Esophagus (PROACTIVE)A Phase 3 Randomized Clinical Trial
https://jamanetwork.com/journals/jamaoncology/fullarticle/2789387Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology–RTOG 1203
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6097832/Intensity-modulated radiotherapy reduces toxicity with similar biochemical control compared with 3-dimensional conformal radiotherapy for prostate cancer: A randomized clinical trial
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.29983Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial
DOI:https://doi.org/10.1016/S1470-2045(10)70290-4Prospective Randomized Study of Intensity-Modulated Radiotherapy on Salivary Gland Function in Early-Stage Nasopharyngeal Carcinoma Patients
DOI: 10.1200/JCO.2007.11.5501







