Prostate Cancer: The Embark Trial
A great option - as long as we don't allow it to replace 1) radiation to the prostate bed and 2) metastasis directed therapy in the oligometastatic setting.
protons101.com
Home to the musings of a radiation oncologist - with a slant on protons and dose and optimizing cancer outcomes.
PREMISE:
My concern is that there are two avenues along the care pathway where radiation use can be avoided or used less often which directly increases the utilization of very high cost drugs. As a specialty we need to constantly promote our value in oncology. These two approaches which de-emphasize proven radiation indication, represent a potential massive shift in healthcare dollars - likely on the order of 1 billion dollars for each of the two decisions we will review. Compare that to the US Medicare Part B dollars spent on radiation oncology of ~1.6 billion for all cancers / all indications. That is my concern.
A few quick notes: US government rad onc spend - ballpark $3-4B - all indications - all centers - all cancers. I think that is reasonable. Part B is around $1.6B (all freestanding technical revenue) - exact recent few year dollars not easy to verify but these are reasonable ballparks I believe. Secondly, the publication for EMBARK is not released. This article is based off what I can find from a recent live presentation. Further, it is not in my daily wheelhouse as it mainly is a systemic treatment, but the dollars are huge.
EMBARK Phase III Trial
Embark data was presented this past weekend in Chicago at the AUA 2023 meeting (ref 1).
The trial looked at treatment options for men with non-metastatic hormone sensitive prostate cancer failing initial therapy - either surgery or radiation - and then if initial treatment was surgery - they needed to fail salvage radiation to the prostate bed. They had CTs and bone scans to look for disease and then could be enrolled on this study - so no clear evidence of metastatic disease at enrollment.
It was a 3 arm trial: 1) Experimental Enzalutamide plus leuprolide, 2) Experimental Enzalutamide monotherapy, and 3) placebo plus leuprolide.
A few highlight slides are shown below.
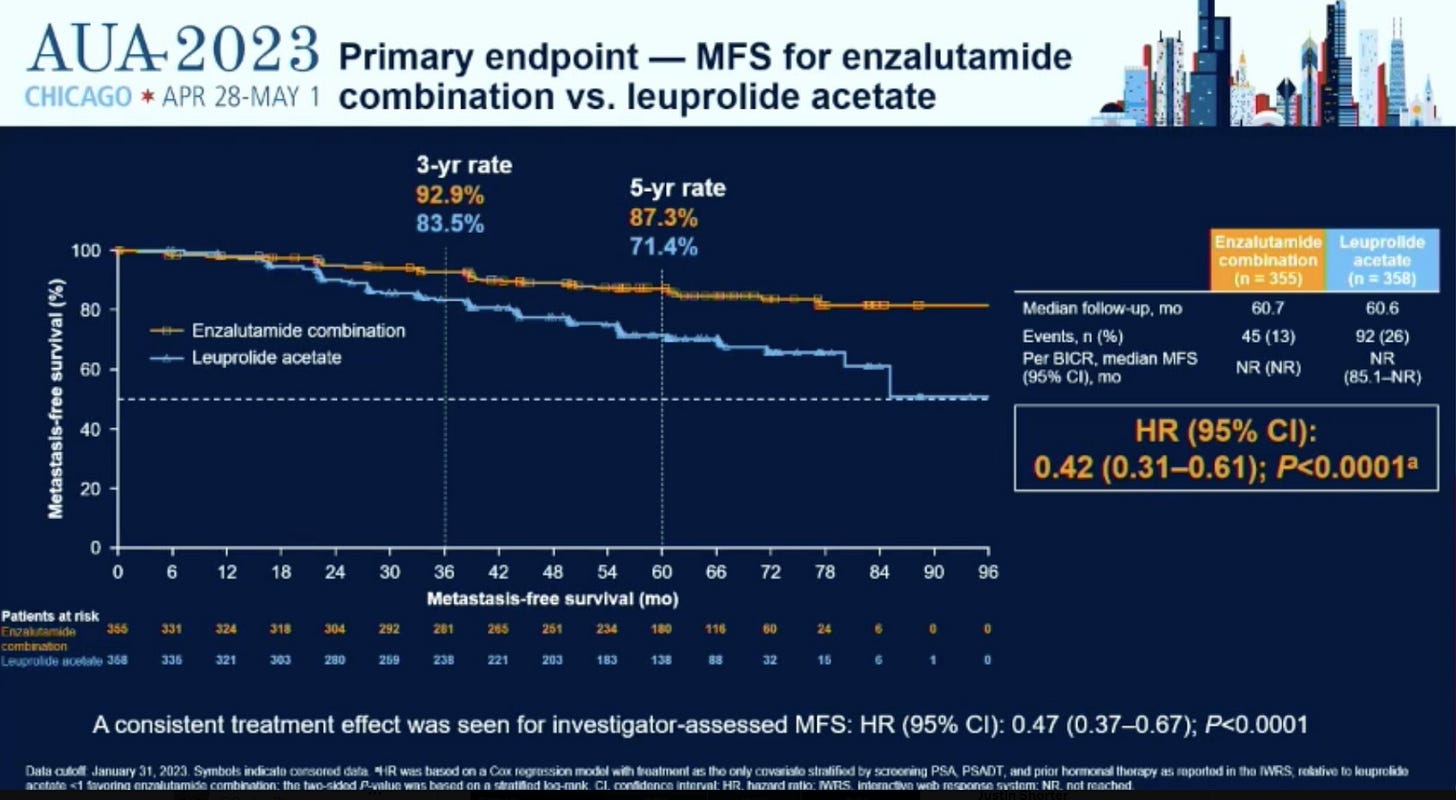
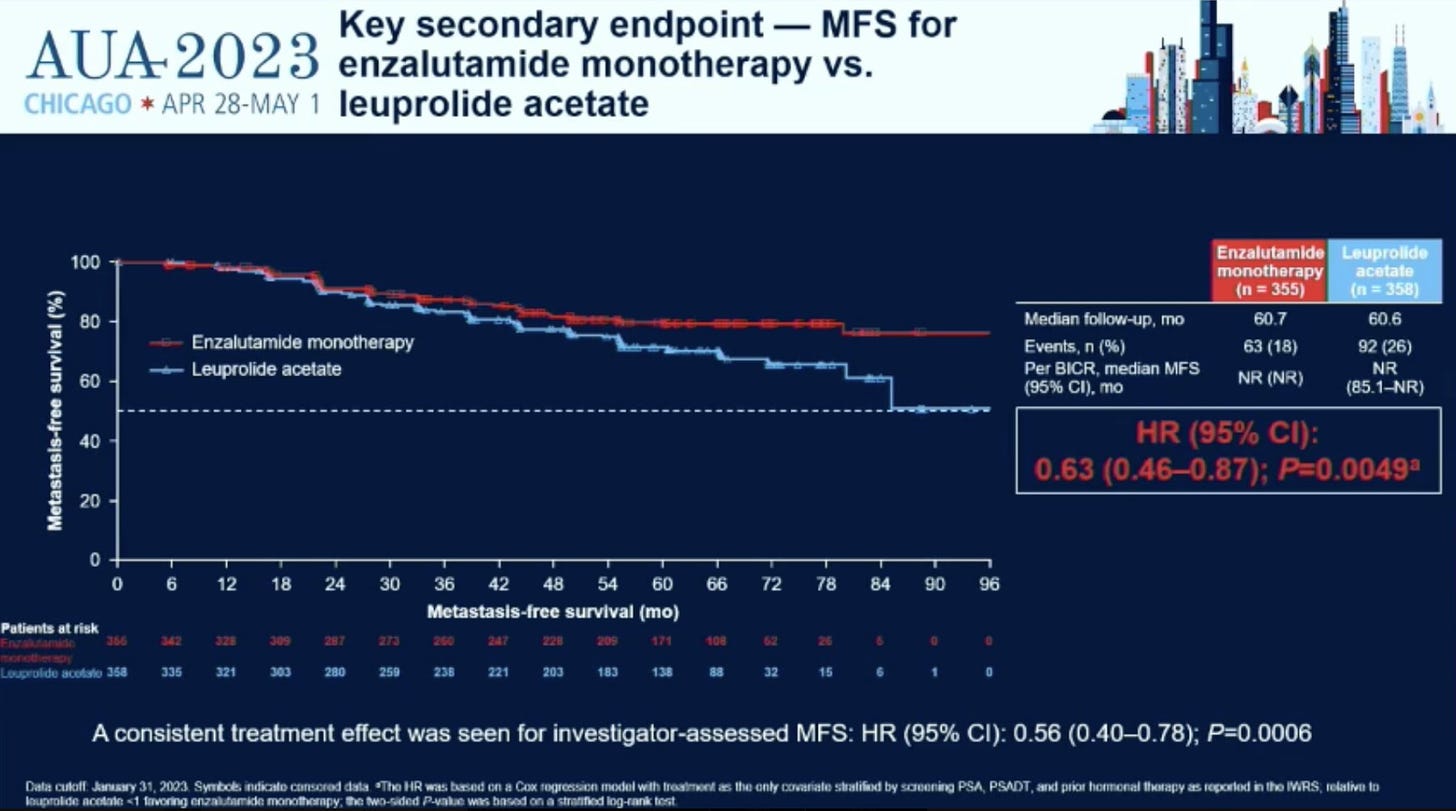
As shown in the curves above, enzalutamide (Xtandi / enza) adds to the benefit of leuprolide acetate (Lupron / ADT) decreasing the rate of metastatic disease in men who have failed local treatment and are at high risk of developing metastatic disease. Further, a simple head to head test of enza vs lupron showed it to be better at preventing metastatic disease.
It’s a pretty clear win in helping to delay at least metastatic sites from appearing. Apparently there is some trend towards an improvement in overall survival but it is not significant currently and any extra treatment comes with extra toxicity. Most benefit was in patients with PSA doubling time >3 months vs. the highest risk cohort with a PSA doubling time < 3 months. 20% of men had PSA greater than 10 and 30% had had prior ADT treatment.
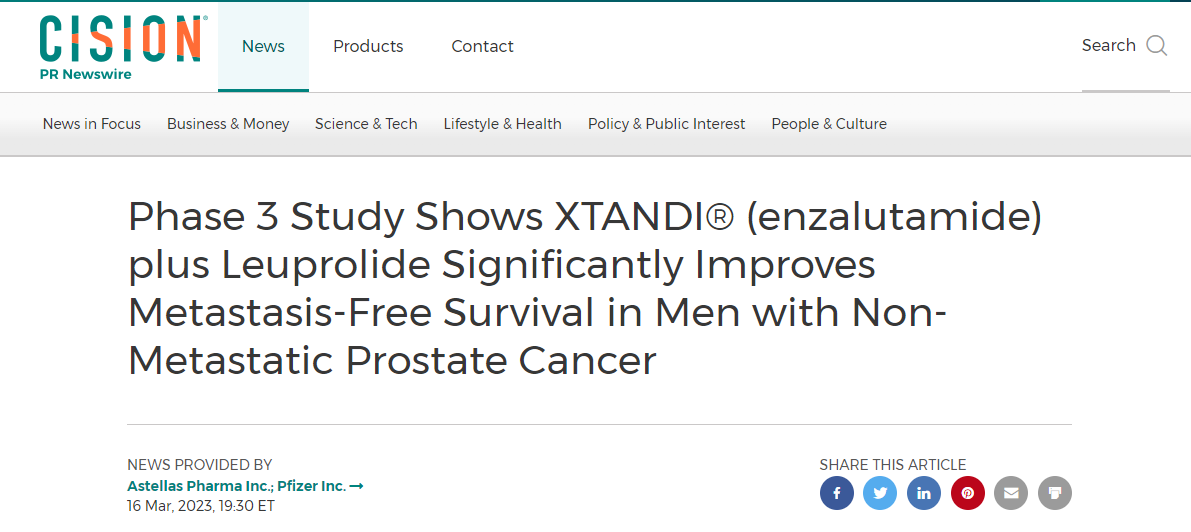
In general this is a win. The Pfizer press release is up as expected as this is a huge business “opportunity”. (ref 2)
Today, we will consider the trial design a bit and look at other approaches that might provide a similar clinical path with far less costs. If this was our only path then I would celebrate it regardless of cost, but as we’ll discuss, I think there are other very appropriate pathways that, at least my concern is, will be under utilized in the wake of a massive marketing campaign in search of revenue.
Here is the official title of the study - it is short and would be easy to include as is in the official press release.
Official Title: A Phase 3, Randomized, Efficacy and Safety Study of Enzalutamide Plus Leuprolide, Enzalutamide Monotherapy, and Placebo Plus Leuprolide in Men With High-Risk Nonmetastatic Prostate Cancer Progressing After Definitive Therapy
Compare that language to paragraph one of the Pfizer press release.
NEW YORK and TOKYO, March 16, 2023 – Pfizer Inc. (NYSE: PFE) and Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., “Astellas”) today announced positive topline results from the Phase 3 EMBARK trial evaluating XTANDI® (enzalutamide) in men with non-metastatic hormone-sensitive prostate cancer (nmHSPC; also known as non-metastatic castration-sensitive prostate cancer or nmCSPC) with high-risk biochemical recurrence (BCR). Patients enrolled in the trial were randomized to one of three study arms: XTANDI plus leuprolide, placebo plus leuprolide, or XTANDI monotherapy. The study met its primary endpoint with a statistically significant and clinically meaningful improvement in metastasis-free survival (MFS) for patients treated with XTANDI plus leuprolide versus placebo plus leuprolide.
Or the title of the press release:
Phase 3 Study Shows XTANDI® (enzalutamide) plus Leuprolide Significantly Improves Metastasis-Free Survival in Men with Non-Metastatic Prostate Cancer
Note: they left out “progressing after definitive therapy.”
This is big business - likely representing BILLIONS of dollars in revenue. This wasn’t sloppy - this was purposeful and details matter. In paragraph one of the press release, in my view they purposefully leave off “progressing after definitive therapy.” That change massively affects the potential market for this drug.
And I can almost guarantee this “error” will show up more broadly in the media without the appropriate qualifiers. Oh look, an example:
Here is some cost data for Xtandi / enzalutamide. Revenue estimates are before this trial was reported (ref 3).
Xtandi's wholesale cost is between $160,000 and $180,000 per patient a year. The companies are expected to have combined revenue from Xtandi of more than $2 billion this calendar year, according to data from Refinitiv.
And that is why I wanted to write this now. Science needs to lead. We can’t let marketing campaigns control our healthcare. Decisions here, related to this trial with their current pricing are large decisions and 5% moves in how patients might be treated are big business and big dollars. We know that big pharma often “struggles” with these types of issues. (I’m trying to be kind - in my assessment they tend to clearly mislead between headlines and actual details - I believe they do it quite clearly here.)
Development of recurrent disease and then to develop metastatic disease is a bad cancer outcome. I think reducing the number of men who have to undergo that path is a very worthwhile endeavor. That said, I want to look at places along the path of prostate cancer management where slight shifts can have large financial impacts - and that concerns me.
Alternate Path One:
Avoidance of Salvage Therapy
Salvage radiation was required, yet not?
25% of men in the trial had surgery alone. Per protocol, salvage radiation to the operative bed is a required component if the patient is eligible. Here is the exact exclusion criteria:
The exclusion criteria are as follows: (seven entries - number 3 is the pertinent one: (3) for patients who had prior RP, a suitable candidate for salvage RT as determined by the investigator per guidelines (eg, ASTRO/AUA, European Association of Urology)
So if suitable for salvage radiation - it was required. (Note: if you just read the headlines - i.e. abstract - it is softer and worded like this):
EMBARK is a randomised, phase 3 study of high-risk patients with nmCSPC, a PSADT of ≤9 months and a screening PSA of ≥2 ng/mL above the nadir after radiotherapy (RT) or ≥1 ng/mL after radical prostatectomy (RP) with or without postoperative RT.
But technically, in the details - it was much stronger and a specific exclusion criteria.
The men on trial have no evidence for distant disease and a rising PSA. That is basically the requirement for salvage treatment and yet, it seems to be that 1 in 3 men post prostatectomy (remove 1/4th of the men on study who received radiation as primary treatment leaving the math 25% / 75% - 1 in 3 men).
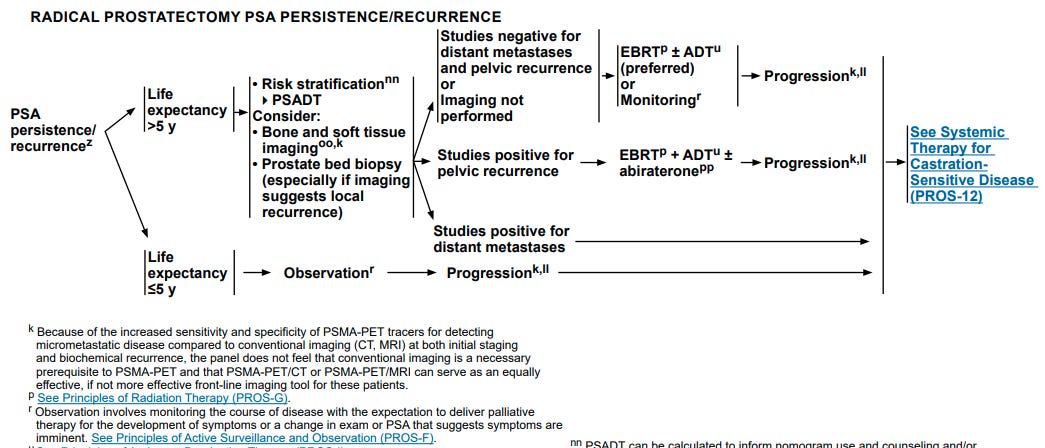
Below is the pertinent NCCN guideline flowchart - you can even blow up the image size and read the pertinent - (r) reference for monitoring criteria - basically a palliative pathway - I’d say far from a 100k per year pathway.
Certainly some men might have ulcerative colitis or other reasons?? to not recommend radiation - but for a full 1/3rd of men to not be candidates for salvage radiation is far too large - probably 6x or 7x the true number.
Is there data or guideline recommendations for salvage treatment?
Yes - that is why it was specifically included in the inclusion / exclusion criteria for this trial and why it is clearly included in the NCCN Guidelines as we see above. Here is a summary of the benefit from the SPPORT trial (ref 4):
Published in Lancet, the SPPORT trial demonstrated that using radiation and 6 months of ADT resulted in a disease free survival rate of 87.4% at 5 years. Note that is actually much stronger than the 87.3% in this trial as in the SPPORT trial, this includes not just the development of metastatic disease but also, a rising PSA.
Why does this matter? Two reasons: which now conflict - Money and patient outcomes.
First the money. Take 100 men who have had prostatectomy and now have a rising PSA with no evidence of metastatic disease. According to the behavior in this trial - where radiation was “required”, 1 in 3 didn’t get that treatment. Let’s move to the real world and say now 1 in 2 men will be treated with salvage radiation who fail prostatectomy.
So our 100 patients is now 50 men. In this scenario, a large percentage of these men pretty quickly will qualify for a $100k per patient per year treatment. If on the other hand 40 of those 50 men were treated with salvage radiation - instead of 50 at risk / eligible for consideration of enza, that number falls to 15. A 3x change in the number of men “at risk” for having benefit of enza or other high dollar approaches.
For each person undergoing salvage radiation, the “business opportunity” for them to benefit from this extra therapy becomes nearly 8 fold less.
Small changes in rates of salvage radiation literally translate to millions if not literally billions of dollars to a pharmaceutical company / companies.
Next let’s look at patient outcomes.
A paper from May 1 in the Journal of Clinical Oncology illustrates my concern: Prostate-Specific Antigen Level at the Time of Salvage Therapy After Radical Prostatectomy for Prostate Cancer and the Risk of Death (ref 5)
It looked at a large database of 25,000 patients and demonstrated that earlier use of salvage radiation was associated with less all cause mortality.
Honestly, I wouldn’t hang my hat on that - I don’t love modeling and big database searches like this, but we have randomized prospective SPPORT data and at least semi-confirming evidence that demonstrates, again, a data-driven rationale to this CLEAR STANDARD OF CARE recommendation. Again, it was included as required in the trial design so no one disagrees with my stance here.
But I haven’t seen this issue mentioned and online and in medical group discussions, I can’t get an answer as to why it was not given, and then I look at the Pfizer press release and I shake my head and I’m concerned. Money often wins these battles.
And again, this type of JUNK happens all the time today and we seem to let it pass. Look at this recent publication for example where the PRIMARY ENDPOINT was altered 19% of the time.
Incidence of Primary End Point Changes Among Active Cancer Phase 3 Randomized Clinical Trials (ref 6)
And arguably worse, 70% of the time, it is NOT mentioned in the write-up - so if you like viewing the world half-full - I guess the EMBARK trial is much better than that - at least this might be considered nuance along the treatment path. I guess.
Alternate Path Two:
Avoidance of Metastasis Directed Therapy
This one shows up in the design of this trial, more than I see in its execution. The design is my issue which largely pre-empts a useful proven clinical path for many of these men. The EMBARK trial sets out to decrease the rate of development of metastasis, which as I state above, is very reasonable. But there is another pathway where we have found that radiation to metastatic disease can create long-term avoidance of ADT.
The data: I’ll cover two, one less optimistic and more optimistic:
The ORIOLE Trial (ref 7): Quoting NCCN which summarizes the first trial quite well:
The ORIOLE phase 2 randomized trial randomized 54 patients with recurrent castration-naïve prostate cancer and 1 to 3 metastases to receive SABR or observation at a 2:1 ratio. The primary outcome measure was progression at 6 months by increasing PSA, progression detected by conventional imaging, symptomatic progression, initiation of ADT for any reason, or death. Progression at 6 months was lower in patients in the SABR arm than in the observation arm (19% vs. 61%; P = .005). The secondary endpoint of PFS was also improved in the patients who received SABR (not reached vs. 5.8 months; HR, 0.30; 95% CI, 0.11– 0.81; P = .002).
The ORIOLE trial is quite small and honestly the benefits of the approach in the trial, to me, are quite limited but with follow-up of 18.8 months the progression free survival endpoint had not been reached the metastasis directed therapy arm meaning a number of men would delay the time to start this more costly whole body hormone related treatment.
STOMP Trial (ref 8): The second trial has much longer follow-up of 5.3 years. Results from this trial are shown below:
Results: The 5-year ADT-free survival was 8% for the surveillance group and 34% for the MDT group (Figure 1, hazard ratio 0.57 [80% CI: 0.38-0.84], log-rank p = 0.06). There was no significant difference in effect for the different stratification factors (interaction test). The 5-year CRPC-free survival was 53% for the surveillance group and 76% for the MDT group (hazard ratio 0.62 [80% CI: 0.35−1.09]; log−rank p = 0.27). At a median follow for survival of 5.3 years (IQR 4.3-6.3), the 5-year overall survival was 85%, with 6 out of 14 deaths attributed to prostate cancer.
So here, over 1 in 4 men were are able to demonstrate a delay of 5 years before we get to the point of needing potential treatments like Xtandi and ADT with the use of metastasis directed therapy.
And if you want to be the very most optimistic, you can quote a “radiation oncology” favorite - the SABR-COMET trial (ref 9)- where 99 patients with a variety of cancers showed a benefit in overall survival with the treatment of metastatic disease.
The SABR-COMET phase 2, international trial randomized 99 patients with controlled primary tumors and 1 to 5 metastatic lesions at 10 centers to standard of care or standard of care plus SABR. Sixteen patients had prostate cancer. After a median followup of 51 months, the 5-year OS rate was higher in the SABR group (17.7% vs. 42.3%; stratified log-rank P = .006), as was the 5-year PFS rate (3.2% vs. 17.3%; P = .001). No differences were seen in adverse events or QOL.
Note: be careful with this one. NCCN prefers to argue it is a progression free survival benefit and the treatment arms in SABR-COMET were imbalanced with respect to cancer histology in favor of the treatment arm.
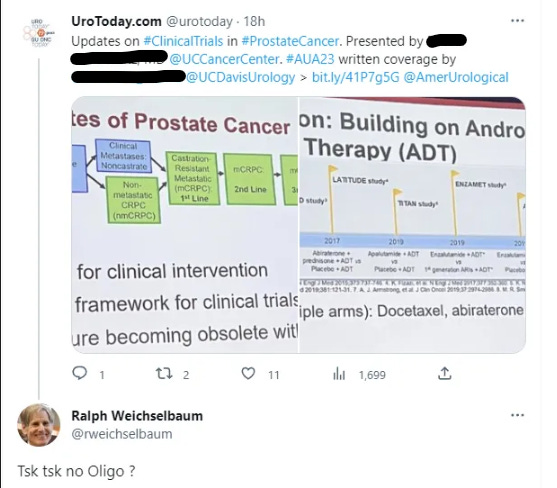
This concern - the lack of oligometastatic disease being considered is at least on the radar of some. A shout out to Dr. Ralph Weichselbaum who I think also is subtly raising a flag of concern below:
It’s a post that talks about the “stages” of prostate cancer and it is - by and large - a systemic only view. It completely negates the Oligometastatic setting where radiation is proven to offer benefit, at a minimum, in delaying a patients course to systemic agents.
Here again, the dollars figures at work benefiting from a reduction in the usage of radiation are massive. My back of envelope estimates are likely - on the order of magnitude for this single indication, the order of magnitude for the US annual Part B expenditure on radiation oncology.
There are scientists doing great work on the topic of trying to better define metastatic disease states - I’ll highlight a thread by Nicholas Zaorsky, MD MS and his colleges who are doing lots of work in the region trying to better define metastatic disease.
Twitter thread on the topic HERE (too much for me to cover today).
But today, my concern is, while we are doing detailed work trying to define and better assign risk stratifications, pharma is often working to remove such nuance in broad swoops.
My Summary:
There are two very subtle paths changes that can make massive revenue / cost differences. These are quite subtle. They are choices to under utilize radiation which has tremendous value - ballpark estimates for salvage treatment is $20k and SBRT for metastatic disease is $5k. And on the other side of that argument is a payment for around $100k in favor of a pharmaceutical approach - per patient, per year of use.
There is clear evidence for this shift / bias / prejudice within the EMBARK trial results with 1 in 3 prostatectomy men not having salvage treatment. And there is clear evidence, in my view, in the press release for wanting to slip slide away details of the indication for broader use. This particular pharmaceutical company did this over and over again in the last few years. Remember the ads for Paxlovid (which I’ve taken) that claimed 88% reduction in hospitalization based on trials with only unvaccinated / never infected patients that ran only after the vast of American’s either had had covid or were vaccinated - and yes, there was a small fine print asterisk - but this is how they choose to run their business. This is my concern. It is a clear consistent pattern. Actions of the past should give us real concern.
The Embark data is great. It demonstrates we have more and more options for the treatment of prostate cancer. But I am concerned with such massive dollar figures on the table that care will be driven into an incredibly high cost option with quite high toxicity - ADT alone is a high toxicity option from my clinical perspective.
Remember in the recent RTOG 0815 trial, the simple addition of “short term” 6 month treatment with ADT increased CTCAE Grade 3 toxicity from 2% to 11%.
And combination treatment with XTANDI is likely worse. It has real toxicity - below is straight from the press release in the disclaimer portion of the release where XTANDI related toxicity is described:
Seizure occurred in 0.5% of patients receiving XTANDI in seven randomized clinical trials.
There have been reports of PRES in patients receiving XTANDI. PRES is a neurological disorder that can present with rapidly evolving symptoms including seizure, headache, lethargy, confusion, blindness, and other visual and neurological disturbances, with or without associated hypertension.
Ischemic Heart Disease In the combined data of four randomized, placebo-controlled clinical studies, ischemic heart disease occurred more commonly in patients on the XTANDI arm compared to patients on the placebo arm (2.9% vs 1.3%).
In the combined data of four randomized, placebo-controlled clinical studies, falls occurred in 11% of patients treated with XTANDI compared to 4% of patients treated with placebo.
Adverse Reactions (ARs) In the data from the four randomized placebo-controlled trials, the most common ARs (≥ 10%) that occurred more frequently (≥ 2% over placebo)
Hypertension: In the combined data from four randomized placebo-controlled clinical trials, hypertension was reported in 12% of XTANDI patients and 5% of placebo patients.
In AFFIRM, the placebo-controlled study of metastatic CRPC (mCRPC) patients who previously received docetaxel, Grade 3 and higher ARs were reported among 47% of XTANDI-treated patients.
Per Pfizer.
RADIATION REPRESENTS TREMENDOUS VALUE:
I write this as a reminder to our field of the value we provide. Many of these men have additional radiation options that were 1) either part of the trial design yet overlooked or 2) not part of the trial design yet supported by strong data. We, as a specialty, need to be proactive and continue to strongly argue for the value that we bring to healthcare. Without a loud persistent voice, we will be beaten with millions and millions of marketing spend promoting far less value oriented drug based approaches - the magnitude on the revenue side for pharmaceutical companies is at least 50% of the US government spend on the entirety radiation oncology. That is my concern.
Special thanks to Todd Scarbrough @toddscarbrough: RadOnc financial guru for help with rad onc spending estimates.
REFERENCES:
LBA02-09 EMBARK: A Phase 3 Randomized Study of Enzalutamide or Placebo Plus Leuprolide Acetate and Enzalutamide Monotherapy in High-risk Biochemically Recurrent Prostate Cancer
https://pubmed.ncbi.nlm.nih.gov/37119051/Phase 3 Study Shows XTANDI® (enzalutamide) plus Leuprolide Significantly Improves Metastasis-Free Survival in Men with Non-Metastatic Prostate Cancer
https://www.pfizer.com/news/press-release/press-release-detail/phase-3-study-shows-xtandir-enzalutamide-plus-leuprolideXTANDI Cost data per year from Reuters
https://www.reuters.com/business/healthcare-pharmaceuticals/us-declines-force-lower-price-cancer-drug-xtandi-2023-03-21/The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01790-6/fulltextProstate-Specific Antigen Level at the Time of Salvage Therapy After Radical Prostatectomy for Prostate Cancer and the Risk of Death
https://ascopubs.org/doi/full/10.1200/JCO.22.02489Incidence of Primary End Point Changes Among Active Cancer Phase 3 Randomized Clinical Trials https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2805005
Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7225913/Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial.
https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.6_suppl.10Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial
https://ascopubs.org/doi/10.1200/JCO.20.00818