Massive Benefit using Radiation in Metastatic Disease!!
Huge reduction in skeletal events, hospitalizations, and yes - perhaps survival - if we include radiation.
So, let’s just say, you sit down and write an article and it is ready to go - pushed to the publish stack - and then “Another One” lands. Do you save that new publication for later or work overtime to create an integrated piece? I opted for the latter, because, as we all know, “You gotta water your plants. Nobody can water them for you.” In that vein, we’ll try to keep this place as up to date as we can, do the work that needs to be done, and cover two great articles within this one piece.
First off, it is quite fun to write up good studies looking at ways where radiation creates benefit in the lives of cancer patients. This is now the third week in a row we highlight something positive and truly powerful with broad indications.
Today we’ll look at a few papers in the metastatic space, where I think radiation continues to demonstrate massive benefit. Exactly how much benefit, you can decide.
The First One:
We’ll start with the first paper released back in September just before ASTRO
Prophylactic Radiation Therapy Versus Standard of Care for Patients With High-Risk Asymptomatic Bone Metastases: A Multicenter, Randomized Phase II Clinical Trial
This is a randomized phase II prospective trial looking at treating asymptomatic high risk lesions with radiation, largely out of Memorial Sloan Kettering, and it simply showed large benefits for the inclusion of radiation - up front and early to avoid skeletal-related events (i.e. things like fracture or an orthopedic procedure).
The trial structure is available via the design document HERE:
What was the basic question?
Does adding radiation to standard of care (without radiation) benefit patients for asymptomatic “high risk” bone lesions? The key here is moving the radiation earlier for “high risk” lesions - not waiting until they become symptomatic, but earlier referrals and treatment for worrisome / high risk lesions. Similar to the concept in oligometastatic trials, but with a unique slant.
The primary outcome was reducing skeletal events. And with it being randomized, they need to define “high risk” for the prospective trial and skeletal related events. Skeletal related events include pathologic fracture, spinal cord compression, orthopedic surgery to bone and or palliative radiation for pain.
What was a high risk lesion?
Bulky disease in bone>2cm, disease in hip, acetabulum, femoral head / neck, shoulder (acromion, glenoid, humeral head), SI joint, long bones>2/3 cortical thickness and junctional vertebral disease in C7-T1, T12-L1, and L5-S1.
Think about that next time you are treating spine disease - the met at T12 was considered higher risk than the met at T10. “Junctional vertebral disease”. (I learn something new daily it seems). From the people I’ve talked to, not many are really going to emphasize this issue but not bad to remember. This definition of “high risk” screams “board exam question”).
How was the radiation given?
Important to note in 2023 - this is NOT necessarily a high dose SBRT trial. Yes, there are options for 24 Gy / 3 or for 24 Gy / 1 there are also 8 Gy / 1 or 30 Gy / 10.
Study Size:
78 patients enrolled with 122 bone metastatic lesions. In the analysis the trial is quite small with 35 in the radiation arm and 36 in the standard of care arm. Arms are pretty well balanced by histology. A few more with hip / SI joint disease in the radiation arm and a few more with >1 lesion in the radiation arm.
What were the results of using radiation in the care plan?
Far less skeletal related events when radiation was part of the plan.
On multivariable regression, study arm (HR, 0.46 for RT arm; 95% CI, 0.25 to 0.83; P 5 .01) and histology (HR, 4.09 for other v breast/prostate; 95% CI, 2.14 to 7.83; P < .001) were associate with outcome.
There were no grade 3 or higher toxicities. 13% did have grade 2 toxicity.
If you are following along pretty closely, you’ll realize that getting radiation simply later could be a cause for this difference - so they looked specifically at that question - did we just delay the use of radiation for these patients?
So they did a secondary analysis and found that even if you removed future palliative radiation from the skeletal-related events, it remained different between arms:
Additional unplanned analyses of the primary outcome were also pursued. When removing SREs related to palliative radiation (ie, including only pathologic fracture, cord compression, and/or surgical intervention for stability), a significant reduction was still observed in the prophylactic RT arm (12% v 0%; P 5 .008).
So the early treatment arm did appear to add more than delay radiation treatment - it appears to have prevented pathologic fracture, cord compression and / or surgical intervention (note, not primary trial goal but via an unplanned analysis so my wording is purposefully cautious).
And maybe?? Survival:
The trial also demonstrated this difference resulting in an overall survival benefit. The P value is quite strong and the curves DO appear to be different in their shape.
This is similar to broader data in a way. If you keep the disease at lower volumes and keep people out of the hospital, they do better. And maybe in the landscape of immunotherapy, this effect will be magnified even further - just a guess at this point, but patterns are beginning to form around this concept and 20 years of data speaks to the point of being more aggressive with disease than we were back in the late 90’s in the metastatic setting.
And via the bisphosphonate data, we know that prevention can affect overall survival. Again, this study is consistent with that body of literature.
And what about other data?
I’ll just point out a few different studies that look at radiation in the metastatic setting. Of course there are some classics, but it’s good to keep up with other new studies as well and see where they are consistent and where they are not.
The CHEERS Phase 2 Randomized Clinical Trial - published in JAMA Oncology back in July of this year.
Here they looked at SBRT to most of the metastatic lesions and saw no clear benefit in survival. It was 99 patients treating patients with metastatic melanoma, renal cell, HN Squamous, urothelial and non-small cell lung cancer.
With a median (range) follow-up of 12.5 (0.7-46.2) months, median PFS was 2.8 months in the control arm compared with 4.4 months in the experimental arm (hazard ratio, 0.95; 95% CI, 0.58-1.53; P = .82). Between the control and experimental arms, no improvement in median OS was observed (11.0 vs 14.3 months; hazard ratio, 0.82; 95% CI, 0.48-1.41; P = .47), and objective response rate was not statistically significantly different (22% vs 27%; P = .56), despite a local control rate of 75% in irradiated patients.
In this trial we see excellent local control and actually, there a non-significant trend towards longer survival of 14.3 months vs. 11.0 months with the addition of radiation - so it trends in the correct direction, but it doesn’t come close to significant with a massive confidence interval. (For comparison in the positive trial for “high risk” lesions, I see about 18 months vs. 11 months in the graphs above for comparison - so a 4 month difference in the radiation addition arm flips the result from not significant to quite significant). Makes sense.
And prior to the release of “Another One”, I wrote this attempting to temper enthusiasm around the overall survival benefit.
How do you reconcile the two?
My guess is like Dr. Willers’ who I think hit the nail on the head with an insightful comment discussing the overall survival difference finding.
Subtle imbalances between arms and quite possibly really good selection of true asymptomatic high risk cases that led to delays or complications or delays in treatment.
Simply stated, historically we haven’t seen this type of effect broadly. A survival benefit did show up in SABR-COMET, but I believe most thought this was due to imbalances in cancer histology in favor of the treatment arm translating to the demonstrated benefit.
Remember in that trial there were 99 patients with a variety of cancers randomized and it showed a benefit in overall survival with the treatment of metastatic disease.
The SABR-COMET phase 2, international trial randomized 99 patients with controlled primary tumors and 1 to 5 metastatic lesions at 10 centers to standard of care or standard of care plus SABR. Sixteen patients had prostate cancer. After a median followup of 51 months, the 5-year OS rate was higher in the SABR group (17.7% vs. 42.3%; stratified log-rank P = .006), as was the 5-year PFS rate (3.2% vs. 17.3%; P = .001). No differences were seen in adverse events or QOL.
So this new study is not one off data, but I think the overall survival benefit question is far from settled.
Then a third trial - similar outcomes to the CHEERS trial above. It trends the same way but again consistent with less effect - the ARTO trial - released September 21st of 2023.
This trial looked at oligometastatic castrate-resistant prostate cancer (less than 3 sites) and randomized patients to abiraterone and prednisone or the same meds with concomitant SBRT to all sites of disease. It enrolled 157 patients. Main results shown below.
Again, a consistent large benefit in progression free survival and maybe hints at OS, but, in my assessment, more consistent with the type of overall survival benefit most would have expected.
Note: Myself, Dr. Willers and the authors of the high-risk study all backing away from leaning into the overall survival difference.
And then… Another One
As of November 15th, that is where the data was. We had the classic COMET-SABR trial with arm imbalances and this trial looking at “high risk” metastatic lesions that showed a huge difference in survival, but many trials showed more muted differences. So, my stance was to be pretty conservative and not bang on the table too much regarding survival. But then in Lancet, this one landed on the 16th of November:
Systemic therapy with or without local intervention for oligometastatic oesophageal squamous cell carcinoma (ESO-Shanghai 13): an open-label, randomised, phase 2 trial
A second phase II trial. This time out of China looking at patients with Esophageal cancer where patients were randomized between systemic therapy or combined systemic and local therapy - local therapy was 89% radiation. The metastatic treatment was delivered to all metastatic sites. The cohort was most metachronous with 80% having 1-2 metastatic lesions. Again, a smaller phase II trial with 104 patients randomized…
but bam.. A large survival benefit lands again!!
Along with the more expected improvement in progression free survival:
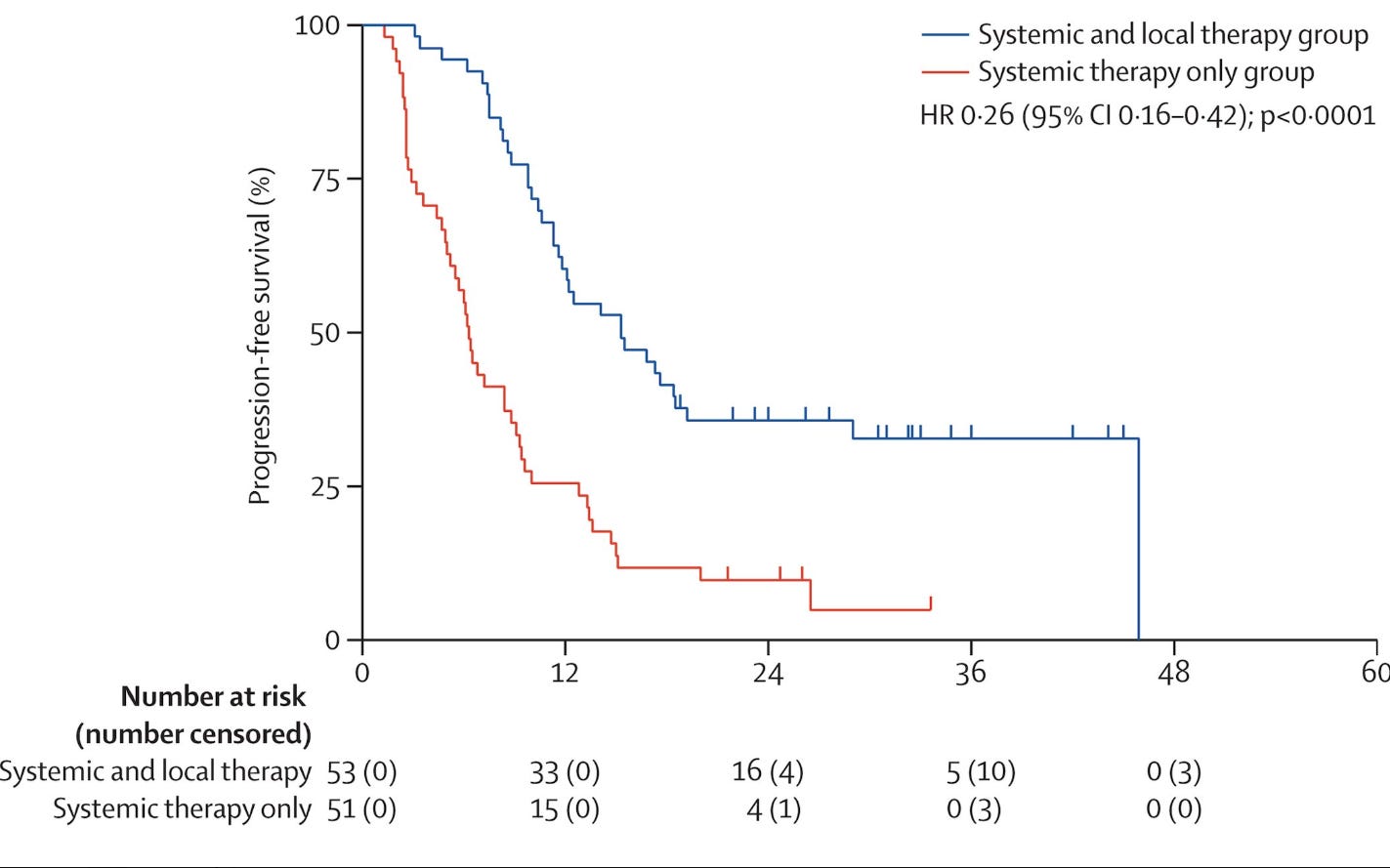
With limited toxicity difference in Gr1 - 2 esophagitis and no difference in Gr3 toxicity. Many were treated with ablative doses but this trial did permit conventional approaches and even surgery or thermal ablation but the point was simple - add in aggressive local treatment and control and survival improves.
Summary:
As usual, I like to back up and look across multiple sites and see if things “make sense”. Today we reviewed four studies - two of which appear to be home runs and two where the trends are in the same direction, just not quite reaching significance. And in the background of all of this new data lies the SABR-COMET trial.
To me, radiation is simply mandated in most of these scenarios. It is lower cost that progression to newer systemic therapies and likely has real potential for an overall survival or - at least - quality of life improvements. You see this benefit in these trials and really across a variety of settings.
Do I 100% trust the overall survival benefit? Not for everything. I think we are still figuring out which locations and which histologies and in which settings benefit the most. But these are now real patterns that are showing up in our data. In fact, I actually disagree with this conclusion in the Lancet article:
Interpretation:
The addition of local treatment for metastases could significantly improve progression-free survival among patients with oligometastatic oesophageal squamous cell carcinoma being treated with systemic therapy. Our findings suggest that combining local and systemic therapy could be a treatment option for patients with oligometastatic oesophageal squamous cell carcinoma, but further support from phase 3 trials is required. (ESO-Shanghai 13 trial)
I think if you consider context across a variety of sites, the data is far stronger than this conclusion. So much upside vs. so little downside - in repeated datasets - in repeated trials - in repeated settings. The lack of radiation integration into a treatment plan, I think, begins to carry real risk of undertreatment for many of these cases.
To me, these studies re-emphasize the role that radiation maintains as a critical component in the treatment of patients with metastatic disease and oligometastatic disease. New drugs and new approaches do NOT allow us to bypass radiation. In fact, I think it is likely that the stronger systemic options have allowed us to see a larger benefit, if we obtain local control of disease.
In this way, it is consistent with our history within breast cancer, not metastatic breast cancer, but the primary up-front role for radiation in the management of the disease. Time and again, we’ve asked whether or not we can drop the radiation now that “control is better” or “we have this new systemic agent” and time and time again, it has been proven that radiation decreases local recurrence. I think we are seeing the same thing here. And until we get to an absolute failure rate of 5% or less, (which is unfortunately a long way off in metastatic disease), we need to remember that radiation maintains its role as a central piece of treatment - creating a hazard rate improvement in both local and often systemic outcomes.
Kudos to the authors and all involved in these four recent trials! Always great to write about positive news on the benefits that radiation can provide to our patients. And remember, if you are bothered by an insurance company, there are now at least 3 prospective trials in the metastatic setting demonstrating an overall survival benefit within different but similar scenarios. So if you think treatment is clinically indicated, push strongly:
Bookmark this page for easy reference. Often as radiation oncologists, we seem to be concerned with finding ways to dismiss the finding in the data. But data is data and what we reviewed today strengthen powerful and broad treatment indications for our field.
There are likely others that should be in this list - if you have one to add to the list, I’d like it to be complete so please email or comment below.
Next week, I think we’ll break the winning streak and look at an article, that at least from my perspective, does what these two articles do not attempt. The one next week reaches too far and over steps the “importance” of the findings. And per all too common for our field, the upcoming article emphasizes systemic options too much in my assessment and thereby undermines the importance and the excellent outcomes with radiation. Oh well, can’t win them all.
Thanks for following along as we continue to discuss the tremendous value of radiation in our search for better. If you made it this far, please consider hitting the like button - it really does help promote the content and it takes less time than it took me to write this - guaranteed.











We are beginning to see the limits of targeted therapy and immunotherapy and all the combinations. At this point we should understand the different prognostic groups for the cancers that respond well to the newer agents (lung, renal, melanoma, breast, prostate, colorectal) and have realistic expectations for the expected survival without SABR. Ex: ESRO/ESTRO classification system,Plichta JCO 2023;14:2546, Keynote 024 etc.